Zegalogue
Generic name: dasiglucagon
Dosage form: injection, for subcutaneous use
Drug class: Glucose elevating agents
What is Zegalogue?
Zegalogue is a prescription medicine used to treat very low blood sugar (severe hypoglycemia) in people with diabetes aged 6 years and older.
It is not known if Zegalogue is safe and effective in children under 6 years of age.
Who should not use Zegalogue?
Do not use Zegalogue if you:
- have a tumor called pheochromocytoma in the gland on top of your kidneys (adrenal gland).
- have a tumor called insulinoma in your pancreas.
Before using Zegalogue
Before using Zegalogue, tell your healthcare provider about all of your medical conditions, including if you:
- have a tumor in your pancreas.
- are allergic to dasiglucagon or any other ingredients in Zegalogue. See the end of this page for a complete list of ingredients.
- have not had food or water for a long time (prolonged fasting or starvation).
- have adrenal insufficiency.
- have low blood sugar that does not go away (chronic hypoglycemia).
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if dasiglucagon passes into your breast milk. You and your healthcare provider should decide if you can use Zegalogue while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements.
How should I use Zegalogue?
- Use Zegalogue exactly how your healthcare provider tells you to use it.
- Read the detailed Instructions For Use that come with your prescription.
- Make sure your caregiver knows where you keep your auto-injectorn or syringe and how to use Zegalogue the right way before you need it.
- Your caregiver must act quickly. Having very low blood sugar for a period of time may be harmful.
- Your healthcare provider will tell you how and when to use Zegalogue.
- After giving Zegalogue, your caregiver should call for emergency medical help right away.
- Once you are able to safely consume food or drink, your caregiver should give you a fast-acting source of sugar (such as fruit juice) and a long-acting source of sugar (such as crackers with cheese or peanut butter).
- If you do not respond to treatment after 15 minutes, your caregiver may give you another dose, if available.
- Tell your healthcare provider each time you use Zegalogue. Your healthcare provider may need to change the dose of your other diabetes medicines.
Zegalogue side effects
Zegalogue may cause serious side effects, including:
- high blood pressure. Dasiglucagon can cause high blood pressure in certain people with tumors in their adrenal glands.
- low blood sugar. Dasiglucagon can cause certain people with tumors in their pancreas called insulinomas to have low blood sugar.
- serious allergic reaction. Call your healthcare provider or get medical help right away if you have a serious allergic reaction including: rash, difficulty breathing, or low blood pressure (hypotension).
The most common side effects in adults include:
- nausea
- vomiting
- headache
- diarrhea
- injection site pain
The most common side effects in children include:
- nausea
- vomiting
- headache
- injection site pain
These are not all of the possible side effects. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Zegalogue?
- Store in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze.
- Zegalogue can also be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 12 months. See example in the Instructions For Use for how to keep track of this 12-month period.
- Do not return Zegalogue to the refrigerator after storing at room temperature.
- Throw away Zegalogue if it has been stored at room temperature for more than 12 months.
- Replace Zegalogue before the expiration date printed on the red protective case.
- Store this medicine in the red protective case it comes in.
Keep all medicines out of the reach of children and pets .
Related/similar drugs
General information about the safe use of Zegalogue.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
Do not use Zegalogue for a condition for which it was not prescribed. Do not give it to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
What are the ingredients in Zegalogue?
Active ingredient: dasiglucagon, provided as dasiglucagon hydrochloride
Inactive ingredients: tromethamine, sodium chloride and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH
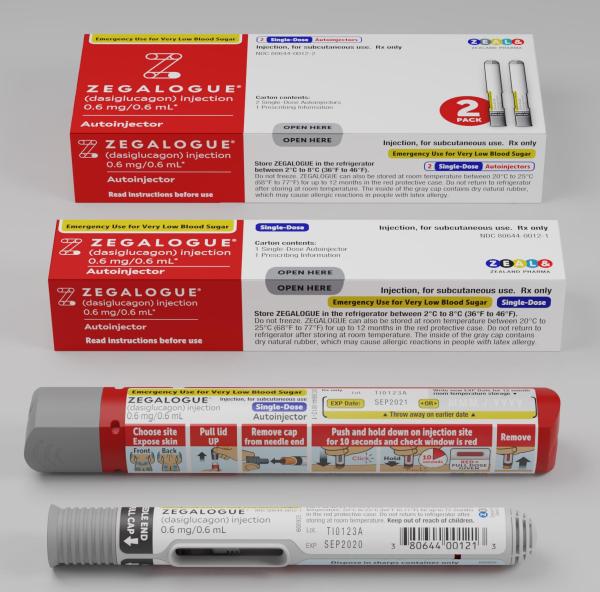
INSTRUCTIONS FOR USE
Important: Zegalogue is used to treat very low blood sugar (severe hypoglycemia) where you need help from others.
Zegalogue contains 1 dose of dasiglucagon in a prefilled autoinjector or prefilled syringe and cannot be reused.
Read and understand this Instructions For Use before an emergency happens.
Show your family and friends where you keep Zegalogue and explain how to use it by sharing these instructions, so they know how to use Zegalogue before an emergency happens.
The inside of the gray cap (autoinjector) or gray needle cover (syringe) contains dry natural rubber, which may cause allergic reactions in people with latex allergy.
Read before injecting Zegalogue
- Do not use Zegalogue if the:
- expiration date has passed
- gray cap (autoinjector) or gray needle cover (syringe) is missing or
- autoinjector or syringe appears damaged
- When opening the red protective case make sure to hold it up straight (with the gray lid or gray cap on top) to avoid dropping Zegalogue.
- Do not remove the gray cap (autoinjector) or gray needle cover (syringe) until you are ready to inject Zegalogue.
- For questions or more information about Zegalogue, call your healthcare provider.
Storage information
- Store in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze.
- Store Zegalogue in the red protective case it comes in.
- Zegalogue can also be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 12 months. See example to the right for how to keep track of this 12-month period.
- Do not return to refrigerator after storing at room temperature.
- Throw away Zegalogue if it has been stored at room temperature for more than 12 months.
- Replace before the expiration date printed on the red protective case.
- Keep Zegalogue and all medicines out of the reach of children.
Before injection
- Choose injection site and expose skin
- Injection sites include:
- Outer upper arms
- Lower abdomen (at least 2 inches from the belly button)
- Front or back of thighs
- Buttocks Roll back any clothing to expose bare skin. Do not inject through clothes.
- Hold the red protective case upright and remove gray lid (autoinjector) or gray cap (syringe).
- Hold the red protective case upright with the gray lid (autoinjector) or gray cap (syringe) on top.
- Pull the gray lid (autoinjector) or gray cap (syringe) up to open.
- Carefully remove Zegalogue from the red protective case without dropping it.
How to inject using Prefilled Autoinjector
Step 1
- Remove the gray cap from needle end.
- Pull the gray cap straight off.
- Do not put your hand or fingers anywhere near the yellow needle guard. Touching the yellow needle guard may cause an accidental needle stick injury.
Step 2
- Push and hold down Zegalogue for 10 seconds and check window is red.
- Push Zegalogue straight down on skin until the yellow needle guard is fully pressed down. You may hear the first click.
- Keep holding Zegalogue down and slowly count to 10 seconds.
- During this time the medicine window will turn red and you may hear a second click.
- Check that the medicine window is red, which means that the full dose has been given.
Step 3
- Remove Zegalogue from injection site.
- Remove Zegalogue by lifting it straight up.
- The yellow needle guard will cover the needle and lock, preventing an accidental needle stick injury.
How to inject using Prefilled Syringe
Step 1
- Remove the gray needle cover
- Pull the gray needle cover straight off. Be careful not to bend the needle.
Step 2
- Pinch the skin and insert the needle
- Gently pinch the skin and insert the entire needle into the skin at a 45° angle.
Step 3
- Give the injection
- After inserting the needle, release the pinched skin and slowly press the plunger rod all the way down until the syringe is empty and the plunger rod stops.
Step 4
- After the plunger rod stops and the injection is complete, carefully remove the needle from the injection site.
After injection
- After you have given the injection, roll the unconscious person on to their side to prevent choking.
- Call for emergency medical help or a healthcare provider right away after you have injected Zegalogue. Even if the injection helps the person to wake up, still call for emergency medical help right away. If the person does not respond after 15 minutes, another dose may be given, if available.
- Once the person is able to safely consume food or drink, give the person a fast-acting source of sugar (such as fruit juice) and a long-acting source of sugar (such as crackers with cheese or peanut butter).
- Replace the used Zegalogue right away so you will have a new auto-injector or syringe in case you need it.
Hypoglycemia may happen again after receiving Zegalogue treatment.
Early symptoms of hypoglycemia may include:
- sweating
- drowsiness
- dizziness
- sleep disturbances
- irregular heartbeat (palpitation)
- anxiety
- tremor
- blurred vision
- hunger
- slurred speech
- restlessness
- depressed mood
- tingling in the hands, feet, lips, or tongue
- irritability
- abnormal behavior
- lightheadedness
- unsteady movement
- inability to concentrate
- personality changes
- headache
If not treated early, hypoglycemia may worsen and the person may have severe hypoglycemia. Signs of severe hypoglycemia include confusion, seizures, unconsciousness, and death.
What other drugs will affect Zegalogue?
Tell your doctor about all your other medicines, especially:
-
indomethacin; or
This list is not complete. Other drugs may interact with dasiglucagon, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
How to throw away (dispose of) Zegalogue
Put your expired or used auto-injector or syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) autoinjectors, loose needles and syringes in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the correct way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used autoinjectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit it.
Do not recycle your used sharps disposal container.
Popular FAQ
What are the signs and symptoms of hypoglycemia?
Low blood sugar happens in people when the level of sugar in their blood gets too low. Signs and symptoms of hypoglycemia (low blood sugar) include:
- feeling hungry
- feeling nervous or worried
- trembling or shaky feeling
- sweating
- dizziness or light-headedness
- sleepiness
- confusion
- passing out (if low blood sugar is left untreated)
More about Zegalogue (dasiglucagon)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: glucose elevating agents
- Breastfeeding
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.