LU W01 Pill: tan, capsule/oblong, 10mm
The pill with imprint LU W01 (Tan, Capsule/Oblong, 10mm) has been identified as Memantine Hydrochloride 5 mg and is used for Alzheimer's Disease. It belongs to the drug class miscellaneous central nervous system agents and is not a controlled substance.
Images for LU W01
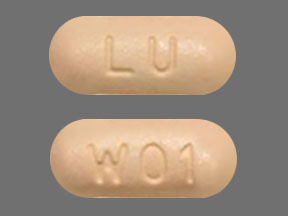
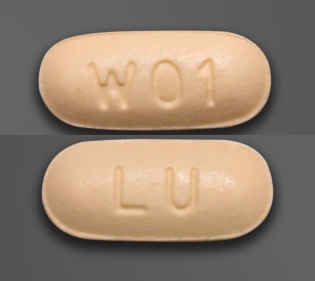

Memantine Hydrochloride
- Imprint
- LU W01
- Strength
- 5 mg
- Color
- Tan
- Size
- 10.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Miscellaneous central nervous system agents
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Lupin Pharmaceuticals, Inc.
- National Drug Code (NDC)
- 68180-0229
- Inactive Ingredients
-
microcrystalline cellulose,
croscarmellose sodium,
FD&C Blue No. 2,
FD&C Yellow No. 6,
hypromelloses,
magnesium stearate,
polyethylene glycol,
magnesium silicate,
titanium dioxide
Note: Inactive ingredients may vary.
See also:
More about memantine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (122)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous central nervous system agents
- En español
Patient resources
Other brands
Professional resources
- Memantine monograph
- Memantine (FDA)
- Memantine ER Tablets (FDA)
- Memantine ER capsules (FDA)
- Memantine Oral Solution (FDA)
- Memantine Tablets (FDA)
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
