93 16 Pill: beige, oval, 20mm
The pill with imprint 93 16 (Beige, Oval, 20mm) has been identified as Nabumetone 750 mg and is used for Osteoarthritis, and Rheumatoid Arthritis. It belongs to the drug class Nonsteroidal anti-inflammatory drugs and is not a controlled substance.
Images for 93 16
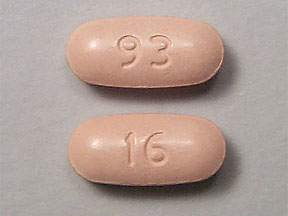



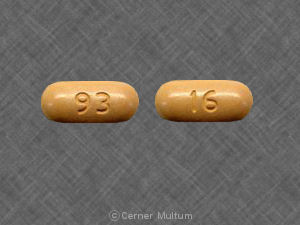
Nabumetone
- Imprint
- 93 16
- Strength
- 750 mg
- Color
- Beige
- Size
- 20.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- Nonsteroidal anti-inflammatory drugs
- Pregnancy Category
- C - Risk cannot be ruled out
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Amneal Pharmaceuticals LLC
- Inactive Ingredients
-
silicon dioxide,
hypromellose 2910 (3 mPa.s),
hypromellose 2910 (5 mPa.s),
microcrystalline cellulose,
polyethylene glycol 400,
polyethylene glycol 6000,
sodium lauryl sulfate,
sodium starch glycolate type A potato,
magnesium silicate,
titanium dioxide,
ferrosoferric oxide,
ferric oxide red,
ferric oxide yellow
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00093-1016 (Discontinued) | Teva Pharmaceuticals USA, Inc. |
| 00115-1658 (Discontinued) | Global Pharmaceuticals |
| 42291-0639 (Discontinued) | AvKare, Inc. |
| 49999-0103 (Discontinued) | Lake Erie Medical and Surgical Supply (repackager) |
| 52959-0656 | H.J. Harkins Company, Inc. (repackager) |
| 54569-5429 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 63874-0687 (Discontinued) | Altura Pharmaceuticals Inc. (repackager) |
| 66267-0529 (Discontinued) | Nucare Pharmaceuticals Inc. (repackager) |
Related images for "93 16"
More about nabumetone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (93)
- Drug images
- Latest FDA alerts (4)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: Nonsteroidal anti-inflammatory drugs
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.