5972 V Pill: white, round, 10mm
The pill with imprint 5972 V (White, Round, 10mm) has been identified as Trihexyphenidyl Hydrochloride 5 mg and is used for Parkinson's Disease, Cerebral Spasticity, and Extrapyramidal Reaction. It belongs to the drug class anticholinergic antiparkinson agents and is not a controlled substance.
Images for 5972 V

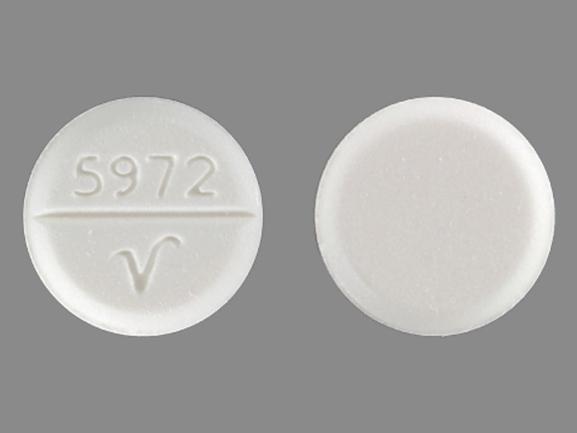


Trihexyphenidyl Hydrochloride
- Imprint
- 5972 V
- Strength
- 5 mg
- Color
- White
- Size
- 10.00 mm
- Shape
- Round
- Availability
- Prescription only
- Drug Class
- Anticholinergic antiparkinson agents
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Qualitest Pharmaceuticals Inc.
- Inactive Ingredients
-
magnesium stearate,
microcrystalline cellulose,
sodium starch glycolate type A potato
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00603-6241 (Discontinued) | Qualitest Pharmaceuticals |
| 62584-0887 (Discontinued) | Amerisource Health Services |
See also:
More about trihexyphenidyl
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (24)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: anticholinergic antiparkinson agents
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
