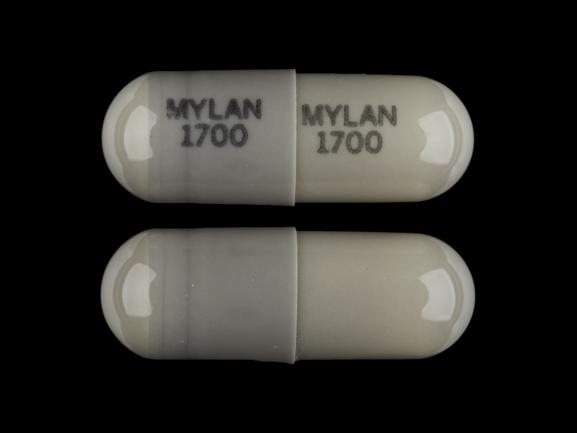
MYLAN 1700 MYLAN 1700 Pill - gray capsule/oblong, 15mm
Pill with imprint MYLAN 1700 MYLAN 1700 is Gray, Capsule/Oblong and has been identified as Nitrofurantoin (Macrocrystals) 100 mg. It is supplied by Mylan Pharmaceuticals Inc.
Nitrofurantoin is used in the treatment of Urinary Tract Infection; Prevention of Bladder infection; Bladder Infection and belongs to the drug class urinary anti-infectives. There is no proven risk in humans during pregnancy. Nitrofurantoin 100 mg is not a controlled substance under the Controlled Substances Act (CSA).
Images for MYLAN 1700 MYLAN 1700



Nitrofurantoin (Macrocrystals)
- Imprint
- MYLAN 1700 MYLAN 1700
- Strength
- 100 mg
- Color
- Gray
- Size
- 15.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Urinary anti-infectives
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Mylan Pharmaceuticals Inc.
- Inactive Ingredients
-
corn starch,
lactose monohydrate,
magnesium silicate,
gelatin,
sodium lauryl sulfate,
titanium dioxide,
ferric oxide yellow,
ferrosoferric oxide,
D&C Yellow No. 10,
FD&C Blue No. 1,
FD&C Blue No. 2,
FD&C Red No. 40,
propylene glycol,
shellac
Note: Inactive ingredients may vary.
Labelers / Repackagers
| NDC Code | Labeler / Repackager |
|---|---|
| 00378-1700 (Discontinued) | Mylan Pharmaceuticals Inc. |
| 51079-0585 (Discontinued) | UDL Laboratories Inc. |
| 43063-0173 (Discontinued) | PDRX Pharmaceuticals Inc. (repackager) |
| 54569-1969 (Discontinued) | A-S Medication Solutions, LLC (repackager) |
| 66267-0156 | Nucare Pharmaceuticals Inc. (repackager) |
See also:
Augmentin
Augmentin is a prescription antibiotic combining amoxicillin and clavulanate to treat bacterial ...
Bactrim
Bactrim (sulfamethoxazole and trimethoprim) is an antibiotic used to treat ear infections, urinary ...
Botox
Botox is used cosmetically to reduce facial lines and wrinkles and for medical purposes for ...
Macrobid
Macrobid (nitrofurantoin) is an antibiotic used to treat urinary tract infections. Includes side ...
Cipro
Cipro (ciprofloxacin) is a fluoroquinolone antibiotic used to treat bacterial infections. Learn ...
Sulfamethoxazole/trimethoprim
Sulfamethoxazole/trimethoprim is used for acne, bacterial infection, bacterial skin infection ...
Amoxicillin/clavulanate
Amoxicillin and clavulanate potassium is a combination antibiotic used to treat bacterial ...
Levofloxacin
Levofloxacin is a fluoroquinolone antibiotic used to treat serious bacterial infections and prevent ...
Ceftriaxone
Ceftriaxone is used for bacteremia, bacterial endocarditis prevention, bacterial infection, bone ...
Ciprofloxacin
Ciprofloxacin is an antibiotic belong to a group of drugs called fluoroquinolones. Learn about side ...
More about nitrofurantoin
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,886)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: urinary anti-infectives
- Breastfeeding
- En español
Patient resources
Other brands
Macrobid, Macrodantin, Furadantin
Professional resources
- Nitrofurantoin monograph
- Nitrofurantoin Capsules (FDA)
- Nitrofurantoin Macrocrystals (FDA)
- Nitrofurantoin Oral Suspension (FDA)
Other brands
Macrobid, Macrodantin, Furadantin
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
