Witness FeLV
This treatment applies to the following species:FELINE LEUKEMIA VIRUS ANTIGEN TEST KIT
I. General Information
WITNESS™ FeLV test is indicated for use when history and/or clinical signs may suggest an infection by feline retroviruses. It will also be particularly recommended prior to an FeLV vaccination, especially in cats belonging to an at risk population.
Ii. Test Principles
WITNESS™ FeLV test is based on Rapid Immuno Migration (RIM)™ technology. FeLV antigen is detected using antibodies directed against the circulating p27 capside protein. Sensitized colloidal gold particles will form a complex with the p27 antigen (FeLV) present in the sample.
The formed complex migrates along the strip. The complex is then captured on a sensitized reaction line where its accumulation causes the formation of a clearly visible pink/red band. Control bands located at the end of the reading window (3) ensure that the test was performed correctly.
III. SAMPLE COLLECTION
● The test can be performed on unclotted, whole blood, anticoagulated with EDTA or heparin, serum or plasma.
● Samples should always be collected with a sterile needle and syringe.
● Hemolysis does not significantly interfere with the test, but strongly hemolyzed samples may partly obscure a weak positive band.
Iv. Sample Storage
Fresh anticoagulated whole blood samples should preferably be tested immediately after collection but not longer than 4 hours after collection, if stored at room temperature. If testing is further delayed, samples should be kept refrigerated (+2°C and 8°C) for up to 4 days. For prolonged storage, samples (serum and plasma only) should be kept frozen (-20°C).
V. KIT CONTENTS
● 10 pouches, each containing 1 test device and desiccant.
● 10 pipettes.
● 1 Saline Buffer dropper bottles (2.2 mL).
● Instructions for use.
VI. PRECAUTIONS
● Do not use components after expiration date.
● Store the test kit at +2°C - 25°C. Do not freeze.
● Use the test immediately after opening the sealed pouch (within 10 minutes).
● Avoid touching or damaging membrane in the sample well or the results window.
● The WITNESS™ device should be placed on a flat, horizontal surface while performing the test.
● Use a separate pipette for each sample.
● Hold pipette and buffer bottle vertically when dispensing sample and buffer respectively.
● Handle all reagents and samples as biohazardous material.
● Saline Buffer contains sodium azide as a preservative.
● For veterinary use only.
Vii. Test Procedure
Important: Allow samples and buffer drops to fall onto membrane at window (1). Do not touch pipette tip, sample or buffer drops, or buffer bottle tip directly to the membrane.
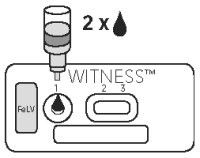
1. SAMPLE APPLICATION
● Tear open a pouch provided and place the test device on a flat horizontal surface.
● Holding the provided pipette vertically, transfer one drop of sample to the sample well (1).

2. BUFFER DISPENSING
● Check that the sample has fully absorbed into the membrane.
● Remove the cap from the buffer bottle, hold it vertically and add two drops of buffer to the sample well (1)
● Leave the test device flat during migration of sample/reagent complex through the reading window.
3. READING TEST
After 10 minutes, observe the presence or absence of pink/red bands in reading windows (2) and (3).
Note:
● It is possible to read the test before 10 minutes if two pink/red bands (respectively in (2) and (3)) are clearly visible.
● The presence of only one band in reading window (3), prior to the end of the development time (10 min), does not mean that the test is complete, as weak samples may appear slower than the control band.
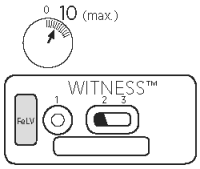
VIII. RESULTS
Validation
Test is validated if a pink/red band is present in the reading window (3).
Interpretation
● Positive. One band in reading window (2), with one band in window (3): sample is positive for FeLV antigen.
● Negative. No band in reading window (2), with one band in window (3): sample is negative for FeLV antigen.
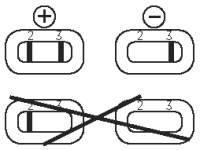
Note:
● No band in control window (3): invalid test.
● A test result should always be interpreted in the context of all available clinical information and history for the cat being tested.
Symbol Descriptions

Zoetis Inc., Kalamazoo, MI 49007, USA
VLN/PCN 190/5028.02
www.zoetis.com
EC REP
ZOETIS FRANCE, 23 Rue Pierre Gilles de Gennes, 69007, Lyon, France
For product information call:
US VMIPS 1-888-963-8471
Canada 1-800-461-0917
40024598
CPN: 3690617.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
