Vetscan Canine Parvovirus Antigen Test Kit, Blot Test
This treatment applies to the following species:I. INTENDED USE
The Vetscan® Canine Parvovirus Rapid Test is a visual and rapid test for the qualitative detection of canine parvovirus antigens in feces. This test is for veterinary in-vitro diagnostic use only.
II. INTRODUCTION
Canine parvovirus is a contagious virus that affects dogs mostly. The intestinal form of the disease causes severe vomiting and hemorrhagic diarrhea in puppies and young dogs1,2.
III. MATERIALS AND REAGENTS PROVIDED
● Test Device. Each sealed pouch contains a test strip in a plastic cassette and a desiccant.
● Sample Dilution Device. Each consists of a sampling swab and 0.7 mL of Extraction Buffer.
● Instructions for Use.
IV. PRECAUTIONS AND WARNINGS
● Parvovirus is infectious.
● The Test Device should remain in the sealed pouch until ready for use.
● The Test Device and the Sample Dilution Device should be used when they are at room temperature (15°-27°C, or 59°-80°F)
● Do not use kit or materials beyond expiration date.
● Do not mix or interchange serials of Vetscan Canine Parvovirus Rapid Test devices.
● Use a new Test Device and a new Sample Dilution Device for each testing. Do not reuse devices.
● Handle samples as biohazardous materials
● Dispose of contents/container in accordance with local/regional/national/international regulations.
● Contains sodium azide as a preservative.
● For veterinary use only.
V. SPECIMEN COLLECTION
● Fresh samples should be used when possible. If samples cannot be used within 24 hours, they should be stored cold (2°-8°C or 36°-46°F) for up to two weeks, or kept frozen before being tested.
● Some fecal samples contain mucous. In order to collect an accurate sample, avoid coating the swab completely with mucous material.
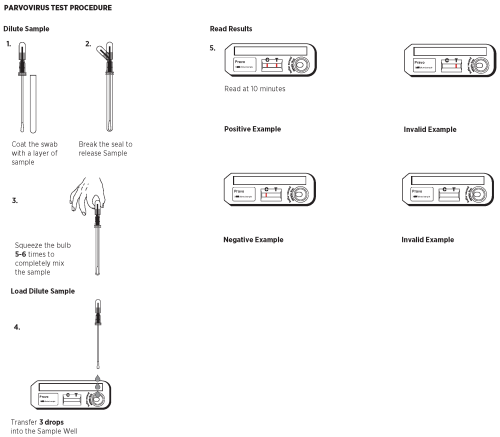
VI. TEST PROCEDURE
1. Remove the sampling swab from the dilution chamber of the Sample Dilution Device. Coat the sampling swab with a thin layer of fecal material or saturate the swab if the sample is liquid. Return the sampling swab back into the dilution chamber.
2. Break the seal of the Extraction Buffer storage bulb located on the top of the Sample Dilution Device by bending the bulb back and forth until the internal blue stick breaks at its base. Squeeze the bulb 5 - 6 times to thoroughly mix the sample with the Extraction Buffer.

3. Remove a Test Device from the protective
pouch and place it on a flat surface.
4. Using the buffer storage bulb and the sampling swab as a pipette, transfer 3 drops (150 µL) of the diluted sample into the round sample well of the Test Device. Hold the pipette vertically to ensure proper sample volume. Start timing.
5. Read the results at 10 minutes. Strong positive results may appear as soon as 1 minute, and weak positive results may take up to 10 minutes to appear. After 10 minutes, discard the Test Device because the results are no longer valid.
VII. INTERPRETATION OF RESULTS
Negative Results
The result is negative when one red to pink line appears in the C (control) area.
Positive Results
The result is positive when two red to pink lines appear. One red /pink line will appear in the T (test) area and one in the C (control) area. A red to pink line with any intensity in the T (test) area should be considered positive. Colored lines may be lighter or darker than each other.
Invalid Results
The test is invalid when no colored line appears in the C (control) area, even if a red to pink line appears in the T (test) area. Repeat the test using a new cassette. Colored lines that appear after 10 minutes are not diagnostic and should be ignored.
VIII. STORAGE
The test kit is to be stored at room temperature (15°-27°C, or 59°-80°F) for the duration of the shelf life. Avoid the test kit being subjected to excessive heat or freezing for a long period of time.
IX. SENSITIVITY AND SPECIFICITY
In an USDA approved study involving 258 samples, the Vetscan Canine Parvovirus Rapid Test correctly identified 93 /96 hemagglutination inhibition (HI) positive canine fecal samples and 157/162 negative canine fecal samples. The discrepant results were resolved by PCR. The sensitivity and the specificity of the test was 96.9% and 96.9%, respectively.
X. REFERENCES
1. Lamm CG, Rezabek GB. Parvovirus infection in domestic companion animals. Vet Clin North Am Small Anim Pract. 2008 Jul;38(4):837-50, viii-ix.
2. Truyen U. Evolution of canine parvovirus--a need for new vaccines? Vet Microbiol. 2006 Oct 5;117(1):9-13.
Manufacturer
SA Scientific, 4919 Golden Quail, San Antonio, TX 78240 USA
VLN/PCN 373/5024.00
1-210-699-8800
Distributor
Zoetis Inc., 333 Portage St., Kalamazoo, MI 49007 USA
1-888-963-8471
www.zoetisus.com
Imported by
Zoetis Belgium S.A., Rue Laid Burniat 1, 1348 Louvain-La-Neuve Belgium
51593100
Presentation: 10 and 20 test kits.
CPN: 3690609.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
