The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
VetScan Canine Flex4 Rapid Test Kit
This treatment applies to the following species:CANINE HEARTWORM ANTIGEN-ANAPLASMA-BORRELIA BURGDORFERI-EHRLICHIA ANTIBODY TEST KIT
The VetScan FLEX4 Rapid Test is for the qualitative detection of antibodies to Anaplasma phagocytophilum, A. platys, Borrelia burgdorferi, Ehrlichia canis, E. chaffeensis, E. ewingii, and Dirofilaria immitis antigens in canine whole blood, serum or plasma.
Intended Use
The VetScan FLEX4 Test Kit is a visual rapid test for the qualitative detection of antibodies to Anaplasma phagocytophilum, antibodies to A. platys, antibodies to Borrelia burgdorferi, antibodies to Ehrlichia canis, antibodies to E. chaffeensis, antibodies to E. ewingii, and Dirofilaria immitis antigens in canine whole blood, serum or plasma. This test is for veterinary use only.
Instructions For Use
This Test is for the detection of antibodies to certain Anaplasma spp., Borrelia spp., and Ehrlichia spp., and Dirofilaria immitis antigens in canine whole blood, serum or plasma samples.
● Samples must be at room temperature (15-27 °C/59-80 °F), before running the assay - DO NOT HEAT.
● Canine whole blood collected in any type of EDTA, heparin, or citrate tubes may be used within one day of collection provided no visual clotting has occurred.
● Do not freeze whole blood or use whole blood that has been frozen. If whole blood is not used within two hours of draw, store refrigerated (2 to 8 °C /35 to 46 °F).
● Serum or plasma, either fresh, previously frozen, or stored at 2 to 8 °C (35 to 46 °F), may be used in this test.
● Serum or plasma may be stored for use up to 7 days at 2 to 8 °C (35 to 46 °F). For longer storage, samples may be frozen at -20 °C (-4 °F) or colder.
● Previously frozen or older serum or plasma samples must be centrifuged at >1600g to remove any particulate material before use.
● Excessive hemolysis may obscure the results.
● EDTA, heparin, or citrate will not affect the results.
PRECAUTIONS AND WARNINGS
● Important: Do not remove device from the pouch until ready for use.
● The Test Device must be used as soon as possible after removing from pouch and within a maximum of 15 minutes.
● For veterinary use only.
● Do not use the components after expiration date.
● The Test Device should be in a horizontal position on a flat surface while the test is performed. The Test Device should not be tilted during the test procedure.
● Use a separate transfer pipette for each patient.
● The chase buffer is not interchangeable from lot to lot.
● Do not use a Test Device from a pouch that is obviously torn or damaged.
● Do not use a Test Device if it appears cracked, broken, or otherwise damaged.
● The kit components must not be frozen.
● Contains Proclin™ 300 and sodium azide as preservatives.
STORAGE
● The test devices and chase buffer can be stored at room temperature or refrigerated (2-27 °C/35-80 °F) and should never be frozen.
● Test devices and chase buffers are stable until expiration when stored at recommended temperatures.
KIT COMPONENTS
1. Test Devices
2. Chase Buffer Bottles
3. Transfer Pipettes
4. Instructions for Use
TEST PROCEDURE
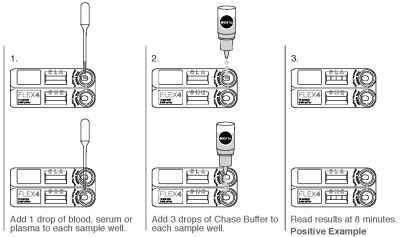
1. Remove the Test Device from the protective pouch and place on a flat surface. Label the Test Device with the patient I.D. or control identification. Only use the provided white space for this purpose. Do not cover up the test name.
2. Gently mix the sample by inverting.
3. Using a provided Transfer Pipette, hold the pipette vertically and dispense one drop of sample (whole blood, serum or plasma) into each sample well.
4. Holding the Chase Buffer Bottle vertically, add 3 drops of the chase buffer into each sample well.
5. Read the results at 8 minutes. Do not read the results after 10 minutes. Colored lines that appear after 10 minutes are not diagnostic and should be ignored.
Interpretation Of Test Results
Positive Results
A test is positive if a colored line appears under the Lyme (L), Anaplasma (A), Heartworm (H), or Ehrlichia (E) areas and a line appears under the Control (C) area in the same row. Any intensity of the Test Line (L, A, H, or E) area should be considered positive. Colored lines may be lighter or darker than each other.
Negative Results
The test lines for each row are negative if only one line appears at the Control line (C) area on that row.
Invalid Results
The test row is invalid if no colored line appears at the Control line (C) area on a given row. Even if a colored line appears in the Test line (L, A, H, or E) areas, there must be a control line (C) on that same row.
FLEX4 Test Procedure
ABAXIS CUSTOMER CARE
In Americas: +1 800 822 2947. Outside Americas: +49 6155 780 210
Manufacturer
SA Scientific, 4919 Golden Quail, San Antonio, TX 78240 USA, VLN/PCN 373/5P23.00
Distributed by: Abaxis, Inc. 3240 Whipple Road, Union City, CA 94587 USA
+1 800 822 2947
www.abaxis.com
ABAXIS Europe GmbH, Bunsenstr. 9-11, 64347 Griesheim, Germany
+49 6155 780 210
For patent information, see www.abaxis.com/about_us/patents.
Learn more about all of our products and services at www.abaxis.com. Abaxis and VetScan are registered trademarks of Abaxis, Inc. © Abaxis 2019.

51012800
510-7039 Rev. C
Presentation: 5 and 25 test kits.
CPN: 1740005.2
Distributed by ZOETIS INC.
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
