The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Solu-Delta-Cortef
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
ACT-O-VIAL® System
prednisolone sodium succinate sterile powder
100 and 500 mg per 10 mL*
For intravenous or intramuscular use
FOR USE IN ANIMALS ONLY
Solu-Delta-Cortef Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
SOLU-DELTA-CORTEF Sterile Powder contains prednisolone sodium succinate which is a salt of prednisolone that is particularly suitable for intravenous or intramuscular injection because it is highly water soluble, permitting administration of relatively large doses in a small volume of diluent. It is especially designed for intravenous use in situations requiring rapid and intense glucocorticoid and/or anti-inflammatory effect; however, it may be used by the intramuscular route in less acute conditions.
*Each mL (when mixed) of these preparations contains:
|
|
100 mg |
500 mg |
|
Prednisolone sodium succinate equivalent to prednisolone |
10 mg |
50 mg |
|
Monobasic sodium phosphate anhydrous |
0.075 mg |
0.075 mg |
|
Dibasic sodium phosphate dried |
0.81 mg |
0.81 mg |
|
Lactose hydrous |
19.6 mg |
19.6 mg |
|
Tyloxapol |
4.9 mg |
4.9 mg |
When necessary, pH was adjusted with sodium hydroxide and/or hydrochloric acid.
Metabolic and Hormonal Effects. Prednisolone, a derivative of hydrocortisone, has greater glucocorticoid activity, greater anti-inflammatory activity, less sodium-retaining effect, and less potassium-losing effect than the parent compound.
The glucocorticoid activity of prednisolone is approximately 4 times that of hydrocortisone and 5 times that of cortisone as measured in experimental animals in terms of liver glycogen deposition, eosinopenic response, and thymic involution.
The anti-inflammatory activity of prednisolone is at least 4 times that of hydrocortisone. SOLU-DELTA-CORTEF exerts an inhibitory influence on the cellular, fibrous, and amorphous components of connective tissue and thereby suppresses the basic processes of inflammation. Vascular permeability is decreased, exudation diminished, and the migration of inflammatory cells markedly impaired.
Solu-Delta-Cortef Indications And Usage
SOLU-DELTA-CORTEF Sterile Powder is indicated for use in situations in which a rapid and intense adrenal glucocorticoid and/or anti-inflammatory effect is necessary. If the intravenous route is impracticable or the need is not so urgent, the intramuscular route may be used.
Inflammatory Conditions. As with the other adrenal steroids, SOLU-DELTA-CORTEF has been found useful in alleviating lameness associated with acute localized and generalized arthritic conditions in horses, dogs, and cats. Treatment is usually required daily or on alternate days, depending on the severity or duration of the condition. Prednisolone sodium succinate has been used successfully to treat bursitis, carpitis, tendinitis, and myositis. Remission of the symptoms may be permanent, or symptoms may recur, depending on the cause and the extent of structural degeneration.
Generalized muscular soreness, stiffness, depression, and anorexia as a result of overtraining, shipping, unusual physical exertion, etc, respond promptly to prednisolone sodium succinate.
The intravenous administration is of particular value in treating acute laminitis (founder) in horses. It is important that the condition be detected early so that therapy may be instituted before there is irreparable damage to the laminae. It may be given at intervals of 12 to 24 hours, depending upon the response. Correction and/or treatment of the etiological factors is imperative and routine local antiphlogistic measures should be employed.
Allergic Reactions. SOLU-DELTA-CORTEF is especially beneficial in treating acute hypersensitivity reactions resulting from treatment with a sensitizing drug or exposure to other allergenic agents. Usual manifestations are anaphylactoid reactions and urticaria. Less severe allergic manifestations, such as atopic and contact dermatitis, summer eczema, and conjunctivitis also may be treated. Response is usually rapid and complete, although in severe cases with extensive lesions, more prolonged adrenocorticoid therapy and other appropriate treatment may be indicated.
Overwhelming Infections with Severe Toxicity. In animals moribund from overwhelmingly severe infections for which specific antibacterial therapy is available (eg, critical pneumonia, peritonitis, endometritis, mastitis), intensive prednisolone sodium succinate therapy may aid in correcting the circulatory defect by counteracting the responsible inflammatory changes, thereby permitting the antibacterial agent to exert its full effect. As supportive therapy, this product combats the stress and improves the general attitude of the animal being treated. All necessary procedures for the establishment of a bacterial diagnosis should be carried out whenever possible before institution of therapy. In the presence of infection, prednisolone sodium succinate should be administered for the shortest possible time compatible with maintenance of an adequate clinical response, and antibacterial therapy should be continued for at least three days after the hormone has been withdrawn. Combined hormone and antibacterial therapy does not obviate the need for indicated surgical treatment.
Shock. For dogs, intravenous SOLU-DELTA-CORTEF is indicated in the prevention and treatment of adrenal failure and shock-like states occurring in association with severe injury or other trauma, emergency surgery, anaphylactoid reactions and elective surgery in poor surgical risks. This hormone is recommended as an adjuvant to standard methods of combating shock, including use of plasma expanders. Because of interrelated physiologic activities, beneficial effects may not be exhibited until all such procedures have been employed. SOLU-DELTA-CORTEF is an invaluable emergency kit drug.
Other Indications. SOLU-DELTA-CORTEF has been found useful as supportive therapy in the treatment of stress-induced exhaustion, rattlesnake bite, toxemia, inflammatory ocular conditions and other stress conditions. Its employment in the treatment of these conditions is recommended as a measure supportive to standard procedures and time-honored treatments and will aid in recovery of the animal.
Contraindications
Except when used for emergency therapy, prednisolone sodium succinate is contraindicated in animals with tuberculosis, Cushingoid syndrome, and peptic ulcer. Existence of congestive heart failure, diabetes, chronic nephritis, and osteoporosis are relative contraindications. In the presence of infection, appropriate antibacterial agents should also be administered and should be continued for at least 3 days after discontinuance of the hormone and disappearance of all signs of infection. Do not use in viral infections.
Warning
Clinical and experimental data have demonstrated that corticosteroids administered orally or parenterally to animals may induce the first stage of parturition when administered during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta and metritis.
Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have resulted in cleft palate in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies, including deformed forelegs, phocomelia, and anasarca.
Precautions
SOLU-DELTA-CORTEF Sterile Powder may suppress systemic manifestations such as fever and also signs of toxemia. In some instances this alteration of the inflammatory reaction may be beneficial; however, it may also mask the signs of infection and tend to facilitate the spread of microorganisms. In infections characterized by overwhelming toxicity, prednisolone sodium succinate therapy in conjunction with indicated antibacterial therapy is effective in reducing mortality and morbidity. It is essential that the causative organism be known and an effective antibacterial agent be administered concurrently. The injudicious use of the adrenal hormones in animals with infections can be hazardous.
Adverse Reactions
The therapeutic use of SOLU-DELTA-CORTEF Sterile Powder is unlikely to cause undesired accentuation of metabolic effects. However, if continued corticosteroid therapy is anticipated, a high protein intake should be provided to keep the animal in positive nitrogen balance. A retardant effect on wound healing has not been encountered, but such a possibility should also be considered when it is used in conjunction with surgery. Euphoria, or an improvement of attitude, and increased appetite are usual manifestations.
Undesirable effects of adrenocorticoid administration are sodium and water retention, potassium loss, glycosuria, hyperglycemia, and polyuria and polydipsia.
Solu-Delta-Cortef Dosage And Administration
Horses: The dosage for horses is 50 to 100 mg as an initial dose. This may be given intravenously over a period of 1/2 to 1 minute, or intramuscularly, and may be repeated in inflammatory, allergic, or other stress conditions, at intervals of 12, 24, or 48 hours, depending upon the size of the animal, the severity of the condition, and the response to treatment. When steroid therapy is to be more prolonged, as in a chronic arthritic condition, DEPO-MEDROL® Sterile Aqueous Suspension containing methylprednisolone or PREDEF® 2X Sterile Aqueous Suspension containing isoflupredone acetate may be injected intramuscularly and continued daily, depending on the severity of the condition and response to treatment.
Dogs: The usual intravenous dose in shock and shock-like states ranges from 2.5 to 5 mg per lb of body weight as an initial dose, followed by equal maintenance doses at 1-, 3-, 6-, or 10-hour intervals as determined by the condition of the patient.
Dogs and Cats: The intramuscular dose in inflammatory, allergic, and less severe stress conditions, where immediate effect is not required, is usually 1 to 5 mg ranging upwards to 30 to 50 mg in large breeds of dogs. This may be repeated in 12 to 24 hours and continued for 3 to 5 days, if necessary. When steroid therapy is to be more prolonged, as in a chronic arthritic or dermal condition, DEPO-MEDROL Sterile Aqueous Suspension may be used. If permanent corticosteroid effect is required, oral therapy with prednisolone tablets may be substituted as soon as possible. When therapy is to be withdrawn after prolonged corticosteroid administration, the daily dose should be reduced gradually over a number of days, in stepwise fashion.
Choice of appropriate concentration of SOLU-DELTA-CORTEF Sterile Powder will help minimize discard of unused drug. It is suggested that the 10 mg/mL formulation be used to treat cats and horses of any size as well as dogs weighing less than 40 pounds. For intravenous treatment of shock in dogs weighing over 40 pounds use the 50 mg/mL formulation. Do not use 50 mg/mL formulation in dogs and cats for intramuscular use.
All intravenous injections should be administered slowly.
Directions For Using The Act-O-Vial System
1. Press down on plastic activator to force diluent into the lower compartment.
2. Gently agitate to effect solution.
3. Remove plastic tab covering center of stopper.
4. Sterilize top of stopper with a suitable germicide.
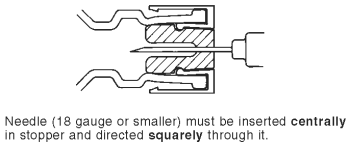
5. Insert 18 gauge or smaller needle squarely through center of plunger-stopper until tip is just visible (as illustrated). Invert vial and withdraw dose.
No additional diluent should be added, and the solution should be injected directly into the vein or muscle. If desired, the solution may be incorporated into the following infusion solutions: Dextrose 5% Injection, Dextrose 5% and Sodium Chloride Injection, Dextrose 10% Injection, Dextrose 10% and Sodium Chloride Injection, Ringer’s Injection, Fructose 10%, and Lactated Potassic Saline Injection (Darrow’s Solution) but must not be added to calcium infusion solutions. If the solution should become cloudy after reconstituting, it should not be used intravenously.
STORAGE CONDITIONS
1. Store unreconstituted product at controlled room temperature 20° to 25° C (68° to 77° F).
Protect from light. Store in carton.
2. Do not store reconstituted product. Use immediately. Discard any unused reconstituted SOLU-DELTA-CORTEF Sterile Powder.
How Supplied
SOLU-DELTA-CORTEF Sterile Powder is available in 10 mL (100 mg or 500 mg/10 mL) ACT-O-VIAL Systems.
NADA 011-593, Approved by FDA
Revised: January 2013
Distributed by: Zoetis Inc., Kalamazoo, MI 49007
PAA036984
CPN: 3690351.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
