SNAP* Combo FeLV Ag/FIV Antibody Test Kit (Canada)
This treatment applies to the following species:Feline Leukemia Virus Antigen-Feline Immunodeficiency Virus Antibody Test Kit
For veterinary use only
SNAP* Combo
The SNAP Combo FeLV Ag/FIV Antibody Test is a rapid immunoassay for simultaneous detection of feline leukemia virus (FeLV) antigen and antibody to feline immunodeficiency virus (FIV) in feline serum, plasma or whole blood. The presence of FeLV p27 antigen is diagnostic for FeLV infection, and the presence of specific antibodies to FIV indicates that a cat has been exposed to FIV and may have an active FIV infection.
Precautions and warnings
● All wastes should be properly decontaminated prior to disposal.
● Do not mix components from kits with different serial numbers.
● Do not use a SNAP device that has been activated prior to the addition of sample.
● Refer to country specific Safety Data Sheet for regional hazard identification.
Kit components
|
Item |
Reagents |
Quantity |
|
1 |
1 bottle, anti-FeLV/FIV Ag: HRPO conjugate (Preserved with gentamicin and ProClin™ 150) |
4.5 mL or 8.5 mL |
|
2 |
SNAP device |
5,15 or 30 |
|
Reagents contained in each device: |
||
|
|
Wash solution (preserved with ProClin™ 150) |
0.4 mL |
|
|
Substrate solution |
0.6 mL |
|
Other components: transfer pipettes, sample tubes and reagent rack |
||
 |
Conjugate - H317/H402/H412/P261/P272/P273/P280/P333+P313/ P363/P501: May cause an allergic skin reaction. Harmful to aquatic life. Harmful to aquatic life with long lasting effects. Avoid breathing mist/vapors. Contaminated work clothing must not be allowed out of the workplace. Avoid release to the environment. Wear protective gloves. If skin irritation or rash occurs: Get medical advice/attention. Wash contaminated clothing before reuse. Dispose of contents/container in accordance with local/regional/national/international regulations. |
|
|
Wash Solution - H317/H319/H402/H412/P261/P264/P272/P273/P280/P305+P351+P338/P337+P313/P333+P313/P363/P501: May cause an allergic skin reaction. Causes serious eye irritation. Harmful to aquatic life. Harmful to aquatic life with long lasting effects. Avoid breathing mist/vapours. Wash thoroughly after handling. Contaminated work clothing must not be allowed out of the workplace. Avoid release to the environment. Wear eye protection/face protection. Wear protective gloves. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. If skin irritation or rash occurs: Get medical advice/attention. Take off contaminated clothing and wash it before reuse. Dispose of contents/container in accordance with local/regional/national/international regulations. |
||
Storage
Store devices and test reagents at 2-8°C. All components must be at room temperature (18-25°C) before running the test-do not heat.
Sample information
● Samples must be at room temperature (18-25°C) before beginning the test procedure.
● Serum, plasma or anticoagulated whole blood (e.g., EDTA, heparin), either fresh or stored at 2-8°C for up to one week, can be used.
● For longer storage, serum or plasma can be frozen (-20°C or colder) and then recentrifuged before use.
● Hemolyzed or lipemic samples will not affect results.
Test procedure
1. Allow all components to equilibrate at room temperature (18-25°C) for 30 minutes before use. Do not heat.
2. Using the pipette provided, dispense 3 drops of sample into a new sample tube.
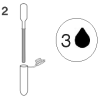
3. Holding the bottle vertical, add 4 drops of conjugate to the sample tube.
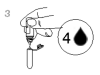
4. Cap the sample tube and mix it thoroughly by inverting it 3-5 times.
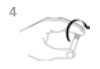
5. Place the device on a horizontal surface. Add the entire contents of the sample tube to the sample well, being careful not to splash the contents outside of the sample well.
The sample will flow across the result window, reaching the activation circle in 30-60 seconds. Some sample may remain in the sample well.
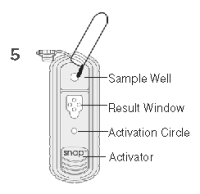
6. When color FIRST appears in the activation circle, push the activator firmly until it is flush with the device body.
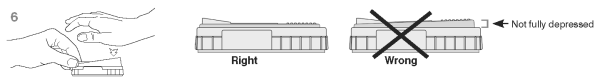
Note: Some samples may not flow to the activation circle within 60 seconds and, therefore, the circle may not turn color. In this case, press the activator after the sample has flowed across the result window.
7. Test results must be read at 10 minutes from the time of activation.
Note: The positive control may develop sooner, but results are not complete until 10 minutes.
Interpreting Test Results
Positive Result
Any color development in the sample spots indicates the presence of FIV antibody or FeLV antigen in the sample.
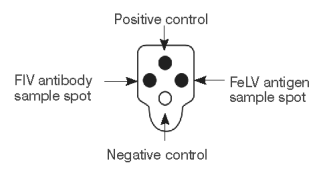
Negative Result
Only the positive control spot develops color.
Reaction With Negative Control
The negative control spot serves as a safeguard against diagnosing false positives, and helps indicate that the assay has been run properly.
Invalid Result
If color in the negative control spot is equal to or darker than the FIV antibody or FeLV antigen sample spot, the test is invalid for that spot.

Invalid results
● Background-If the sample is allowed to flow past the activation circle, background color may result. Some background color is normal. However, if colored background obscures the test result, repeat the test.
● No color development-If the positive control does not develop color, repeat the test.
Idexx Snapshot Dx* Analyzer
Test results can also be read using the SNAPshot Dx analyzer. A complete description of how to enter patient data and read test results using the SNAPshot Dx analyzer can be found in the SNAPshot Dx Analyzer Operator’s Guide.
Symbol descriptions

IDEXX Technical Support
USA/Canada: 1 800 248 2483 • idexx.com
Australia: 1300 44 33 99 • idexx.com.au
Europe: idexx.eu
VLN/PCN: 313/502A.02
*SNAP and SNAPshot Dx are trademarks or registered trademarks of IDEXX Laboratories, Inc. or its affiliates in the United States and/or other countries.
Patent information: idexx.com/patents.
© 2024 IDEXX Laboratories, Inc. All rights reserved.
IDEXX, One IDEXX Drive, Westbrook, Maine 04092 USA
Distributor: IDEXX Canada, 3044 Bloor Street, Toronto, ON M8X 2Y8 Canada
idexx.com
06-01482-20
CPN: 1243004.11
1345 DENISON STREET, MARKHAM, ON, L3R 5V2
| Technical Service: | 800-248-2483 | |
| Telephone: | 800-667-3411 | |
| Order Desk: | 800-248-2483 | |
| Fax: | 905-475-7609 | |
| Website: | www.idexx.ca | |
| Email: | CAGInsideSalesAssociateCND@idexx.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
