NexGard COMBO (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
Esafoxolaner, eprinomectin and praziquantel
Topical solution for cats
VETERINARY USE ONLY
DIN 02511231
DESCRIPTION: NEXGARD COMBO is a spot-on solution for cats. The active ingredients are esafoxolaner (an ectoparasiticide of the isoxazoline family), eprinomectin (an endectocide of the macrocyclic lactone class) and praziquantel (a synthetic isoquinoline-pyrazine derivative endoparasiticide).
INDICATIONS: NEXGARD COMBO topical solution for cats, is indicated for:
- the treatment and control of flea (Ctenocephalides felis) infestations by killing adult fleas,
- the treatment and control of Ixodes scapularis (Blacklegged Ticks) and Amblyomma americanum (Lone Star Ticks),
- the treatment of ear mites (Otodectes cynotis),
- the prevention of heartworm disease (Dirofilaria immitis),
- the treatment and control of intestinal cestode infections caused by the adult tapeworms Dipylidium caninum and Echinococcus multilocularis,
- the treatment and control of intestinal nematode infections caused by adult Toxocara cati; in cats and kittens 8 weeks of age and older.
Dosage and Administration
NEXGARD COMBO is applied topically on a monthly basis as specified below.While an effective treatment for its labeled parasite indications as a single dose (see Efficacy), repeat monthly dosing may be used to prevent re-infestation/infection based on the epidemiology and regional risk of the specific labeled ecto/endoparasite.
Prevention of heartworm disease by killing Dirofilaria immitis larvae should start within 1 month after the first expected exposure to mosquitoes and should be continued until at least 1 month after the last exposure to mosquitoes.
Select the appropriate applicator size for the weight of the cat and administer the entire contents of the unit dose applicator topically, on the neck between the base of the skull and the shoulder blades, as specified in the following table:
|
Cat weight (kg) |
Volume of unit dose (mL) |
Esafoxolaner (mg) |
Eprinomectin (mg) |
Praziquantel (mg) |
|
< 2.5 kg |
0.3 |
3.60 |
1.20 |
24.9 |
|
2.5 - 7.4 kg |
0.9 |
10.8 |
3.60 |
74.7 |
|
> 7.4 kg |
Appropriate combination of applicators |
|||
The dose ranges are 1.44 - 4.24 mg/kg for esafoxolaner, 0.48 - 1.44 mg/kg for eprinomectin and 10 - 29.9 mg/kg for praziquantel.
NEXGARD COMBO should be applied such that the risk of the treated cat, nursing kittens, other household cats, or other pets, licking or coming in contact with the medication is minimized (see CAUTIONS.
Administration to queens should be delayed until at least the 7th day post-partum.
Method of administration:
As shown below, 1. use a pair of scissors to cut the blister along the dotted line, and then pull back the blister backing. Remove the applicator from the package and hold it upright. 2. Pull back the plunger slightly, 3. twist and pull off the cap. 4. Part the cat’s fur on the midline of the neck, between the base of the skull and the shoulder blades until the skin is visible. Place the tip of the applicator on the skin and apply the entire content directly onto the skin in one spot. If the weight of the cat requires a second application, apply the contents in the same manner as described above, in the same location.
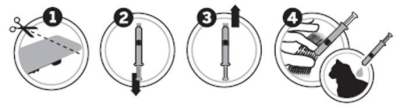
CAUTIONS: NEXGARD COMBO is for topical, spot-on application only. Do not administer orally. Cats may salivate excessively if administered orally or ingested by licking/grooming the application site (see ANIMAL SAFETY). Apply NEXGARD COMBO to the skin where the cat cannot lick it off, such as the dorsal area of the neck or between the shoulder blades. Animals should not lick each other following treatment. Keep out of the cat’s eyes.
Do not wash the cat following product application because the effectiveness of the product under these circumstances has not been tested. Oiliness, wetness or deposits may be observed at the application site for up to 24 hours after administration.
The safety of NEXGARD COMBO has not been evaluated in breeding male (tom) cats.
The safety of NEXGARD COMBO in kittens less than 8 weeks of age has not been evaluated.
Esafoxolaner is a member of the isoxazoline class. This class has been associated with neurological adverse reactions including tremors, ataxia, and seizures. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of Neurological disorders. Use with caution in cats with a history of neurological disorders.
Ticks and fleas need to start feeding on the host to become exposed to esafoxolaner, therefore the risk of transmission of parasitic borne diseases cannot be excluded.
To reduce re-infestation from emergence of new fleas, it is recommended that all animals in a household be treated. All stages of fleas can infest the cat’s basket, bedding and resting areas such as carpets and soft furnishings.
Cats in areas endemic for heartworm, or those which have travelled to endemic areas, may be infected with adult heartworms. It is recommended that all cats, living in areas endemic for heartworm, should be tested for existing infection before being treated with NEXGARD COMBO for heartworm prevention.
For the treatment and control of Amblyomma americanum, the time to achieve >90% effectiveness is 72 hours.
Following treatment of Otodectes cynotis, the ears may need to be cleaned of debris.
For use in cats with or at risk for mixed infections and infestations by cestodes, nematodes and ectoparasites. The product is indicated when all three groups of parasites are targeted at the same time.
Warnings
● Keep out of reach of children.
● When handling the product, wear disposable latex or nitrile gloves to prevent accidental topical exposure.
● Wash hands after application.
● Avoid handling the treated animal and contact with the application site until the application site is no longer noticeable following treatment.
● Recently treated cats should not sleep with their owners until the treated area is no longer noticeable.
● NEXGARD COMBO can cause eye irritation. In case of accidental eye exposure, flush eyes thoroughly with water.
● In case of accidental ingestion, or if skin/eye irritation occurs, seek medical attention immediately.
Adverse Reactions
Although not all adverse reactions are reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. The reported adverse events, listed below by body system in decreasing order of frequency, were observed very rarely (in less than 1 animal per 10,000 treated):
Digestive tract disorders: hypersalivation, vomiting, diarrhea
Systemic disorders: lack of efficacy, anorexia, lethargy, death
Application site disorders: application site hair change, application site pruritus
Neurological disorders: ataxia, muscle tremor
In a multi-centred USA field study evaluating the efficacy of NEXGARD COMBO when administered topically against flea infestations and flea allergy dermatitis in 244 cats once monthly for 3 months, the adverse reactions reported unreflective of causality and occurring in more than one percent of the cats were: emesis (6.6%), application site hair change (3.7%), anorexia (2.9%), lethargy (2.5%), trauma NOS (2.5%), bacterial skin infection (1.6%), pruritus (1.6%), sneezing (1.6%), death (1.2%), desquamation (1.2%), diarrhea (1.2%), epiphora (1.2%), hypersalivation (1.2%) and hyperthermia (1.2%).
There were 3 deaths in the NEXGARD COMBO group and 2 deaths in the positive control group. Of the three deaths reported in the NEXGARD COMBO group, one cat died 4 days after the second treatment. The cat was diagnosed with restrictive cardiomyopathy on post mortem examination. The second cat, an 18 week old kitten was found dead 20 days after the third treatment. On post mortem examination, the kitten had congestive heart failure secondary to cardiomyopathy. The third cat had vomiting, decreased appetite, jaundice and increased liver enzymes. Findings at necropsy were pancreatitis, cholangitis and enteritis with severe hepatic lipidosis. The two positive control cats were euthanized, one after it was paralyzed and the second when it was diagnosed with an esophageal mass. Product causality could not be confirmed in the cases with fatal outcomes.
Clinical Pharmacology
Mode of Action:
Esafoxolaner is the active (S)-enantiomer of afoxolaner and acts as an antagonist at ligand-gated chloride channels, in particular those gated by the neurotransmitter GABA (gamma-aminobutyric acid). Isoxazolines, among the chloride channel modulators, bind to a distinct and unique target site within the insect GABACls, thereby blocking pre- and post-synaptic transfer of chloride ions across cell membranes. Prolonged esafoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines. The selective toxicity of afoxolaner between insects, acarines and mammals may be inferred by the differential sensitivity of the insects and acarines’ GABA receptors versus mammalian GABA receptors. Fleas and ticks are eliminated in 24 and 48 hours respectively after treatment.
Eprinomectin binds selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve or muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarisation of the nerve or muscle cell, resulting in paralysis and death of the parasites.
Praziquantel is rapidly absorbed via the surface of the parasites and affects membrane permeability in cestodes, influencing divalent cation fluxes, particularly calcium ion homeostasis, which is thought to contribute to the rapid muscle contraction and vacuolization. This results in severe damage to the parasite integument, contraction and paralysis, disruption of metabolism and finally leads to death and expulsion of the parasite. Disintegrated and partially digested fragments may occasionally be seen in the feces.
Pharmacokinetics:
Esafoxolaner is systemically absorbed from the topical application site, reaching a maximum concentration in plasma in a median time of 7 days after application. Esafoxolaner is slowly eliminated from plasma (t1/2 = 22 to 29 days) and excreted in feces and urine.
Eprinomectin is systemically absorbed from the topical application site, reaching a maximum concentration in plasma between 8 and 48 hours after application. Eprinomectin is slowly eliminated from plasma (t1/2 = 10 days) and excreted in feces.
Praziquantel is systemically absorbed from the topical application site, reaching a maximum concentration in plasma between 2 and 32 hours after application. Praziquantel is slowly eliminated from plasma (t1/2 = 4 - 10 days) and excreted in urine.
The pharmacokinetic profiles of esafoxolaner, praziquantel and eprinomectin are not affected by co-administration.
EFFICACY:
Treatment and control of flea infestations:
In two controlled laboratory studies, conducted in the USA and South Africa, NEXGARD COMBO used as a single-dose topical treatment was effective and provided ≥95% efficacy against fleas (C. felis), one month after application.
In a US field trial, NEXGARD COMBO also showed ectoparasiticidal effects against both adult fleas and the viability of the flea eggs. Flea egg viability was reduced 8 days after treatment until day 31. Cats with pre-existing signs of flea allergy dermatitis showed improvement in alopecia, miliary dermatitis, excoriations, scaling and erythema as a direct result of eliminating the flea during the study.
Treatment and control of tick infestations:
In two controlled laboratory studies, NEXGARD COMBO demonstrated that a single topical monthly administration, is well tolerated and efficacious (>95% effective through day 32) against Blacklegged ticks (I. scapularis).
Additionally, the NEXGARD COMBO treated group, had in both studies, statistically significant differences for dead tick counts when compared with the placebo control group, at all-time points (P<0.0005 and P≤0.0234 respectively).
In two controlled laboratory studies, NEXGARD COMBO demonstrated that a single topical monthly administration, is well tolerated and efficacious (>90% effective through day 33) against Lone Star ticks (A. americanum). Additionally, the NEXGARD COMBO treated group, had in both studies, statistically significant differences for dead tick counts when compared with the placebo control group, at all-time points (P<0.0001).
Treatment and control of ear mites:
In two controlled laboratory studies and a field study conducted in Greece and Hungary, administration of NEXGARD COMBO at the recommended treatment dose is effective (percentage of efficacy > 96.5%) in the treatment of Otodectes cynotis infestations in cats.
Heartworm Prevention:
In two controlled laboratory studies, administration of a single dose of NEXGARD COMBO, at the minimum recommended dose, was well-tolerated and provided 100% efficacy in the prevention of induced heartworm (Dirofilaria immitis) infection, in cats.
Treatment and control of nematode infestations:
In two controlled laboratory studies, administration of a single dose of NEXGARD COMBO, at its minimum dose, is highly efficacious and safe for the treatment of Toxocara cati. The percentage efficacy in both laboratory studies was 98.8-100% (P<0.0001).
Treatment and control of cestode infections:
In a controlled laboratory study, administration of a single topical dose of NEXGARD COMBO, at the recommended minimum dose, provided 100% effectiveness against an experimental infection of the adult tapeworm (Echinococcus multilocularis).
In two controlled laboratory studies, administration of a single topical dose of NEXGARD COMBO, was greater than 97% effective for the treatment of the tapeworm Dipylidium caninum in cats.
ANIMAL SAFETY:
Margin-of-safety study in kittens:
Safety of NEXGARD COMBO has been demonstrated at 1X, 3X and 5X the maximum topical dose in 4 male and 4 female healthy kittens per group. The kittens were aged 8-9 weeks at the beginning of the study, and were treated 6 times at four-week intervals. At 5X the maximum dose, a single adverse neurological reaction that included hypoactivity, increased respiratory rate, dilated pupils, dorsal recumbency, ataxia, inability to stand, mild tremors and hypothermia was observed 8 hours after the third treatment in one male cat and it was reversible with supportive treatment. Mild pupillary dilation was observed in all treatment groups.
Oral administration study in kittens:
In case of an accidental oral exposure, a study to determine the safety of NEXGARD COMBO in 7-9 week old kittens administered once orally at the maximum label dose was conducted. All 8 treated cats immediately exhibited hypersalivation after oral administration. No other reaction to treatment or abnormal health observations were observed during the 14 day study. No treatment-related changes were observed in any of the hematology, plasma chemistry or urinalysis parameters assessed.
Pregnancy and lactation:
A negative controlled, blinded, randomized safety study was conducted on twenty-nine adult queens treated topically with NEXGARD COMBO every 28 days during the pre-mating, mating, pregnancy, and lactation periods, until 56 days post-partum, There were 10 control queens, 9 queens in the 1x group and 10 queens in the 3x group. The pregnancy rate was 90% in the control and 3 x groups and 77% in the 1x group. The difference in pregnancy rates between the groups was not statistically different. The mean number of kittens born alive to the queens in each group was 3.7 for the control group, 5.1 for the 1x group and 5.7 for the 3x group. The difference between the control and 3x group was statistically significant (p=0.0150). One kitten in the 3x group born with an open fontanelle was euthanized. Eighty-one percent of the control kittens were weaned compared to 86% of kittens in the 1x group and 98% of kittens in the 3x group. The number of weaned kittens between the control group and the 3x group was significantly different (p=0.0134). Vision, hearing and testicle descent were not evaluated. NEXGARD COMBO was demonstrated to be safe with no serious adverse effects when used in non-pregnant, pregnant and lactating queens, for the queens and their offspring, at the maximum exposure topical dose as well as at 3x the maximum exposure dose.
Storage
Store at temperature between 15-30°C. Keep the unused applicator in the blister and in the outer carton in order to protect from light. Opened applicators should be disposed of immediately.How Supplied
NEXGARD COMBO is presented in unit dose syringe-shaped applicators containing 0.3 mL or 0.9 mL solution, closed with a cap and placed in individual plastic blisters.The 0.3 mL applicators are available in cartons of 1, 3, 4, 6 or 15 applicator(s) of 0.3 mL each.
The 0.9 mL applicators are available in cartons of 1, 3, 4, 6 or 15 applicator(s) of 0.9 mL each.
Not all pack sizes may be marketed.
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
NEXGARD COMBO™ is a trademark of Boehringer Ingelheim Animal Health France, used under license.
177032-002
CPN: 1182172.2
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
