Enroflox 100
This treatment applies to the following species: Company: Norbrook
Company: Norbrook
(enrofloxacin)
100 mg/mL Antimicrobial Injectable Solution
Approved by FDA under ANADA # 200-495
For Subcutaneous Use In Beef Cattle And Non-Lactating Dairy Cattle
For Intramuscular Or Subcutaneous Use In Swine
Not For Use In Female Dairy Cattle 20 Months Of Age Or Older Or In Calves To Be Processed For Veal
Enroflox 100 Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
Federal (U.S.A.) law prohibits the extra-label use of this drug in food-producing animals.
To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.
PRODUCT DESCRIPTION:
Enroflox® 100 is a sterile, ready-to-use injectable antimicrobial solution that contains enrofloxacin, a broad-spectrum fluoroquinolone antimicrobial agent. Each mL of Enroflox 100 contains 100 mg of enrofloxacin. Excipients are L-arginine base 200 mg, n-butyl alcohol 30 mg, benzyl alcohol (as a preservative) 20 mg and water for injection q.s.
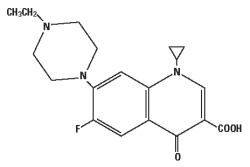
CHEMICAL NOMENCLATURE AND STRUCTURE:
1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid.
Enroflox 100 Indications
Cattle - Single-Dose Therapy: Enroflox 100 is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in beef and non-lactating dairy cattle; and for the control of BRD in beef and non-lactating dairy cattle at high risk of developing BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis.
Cattle - Multiple-Day Therapy: Enroflox 100 is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni in beef and non-lactating dairy cattle.
Swine: Enroflox 100 is indicated for the treatment and control of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae. Enroflox 100 is indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed.
Dosage and Administration
Enroflox 100 provides flexible dosages and durations of therapy.
Enroflox 100 may be administered as a single dose for one day for treatment and control of BRD (cattle), for treatment and control of SRD or for control of colibacillosis (swine), or for multiple days for BRD treatment (cattle). Selection of the appropriate dose and duration of therapy for BRD treatment in cattle should be based on an assessment of the severity of the disease, pathogen susceptibility and clinical response.
Cattle:
Single-Dose Therapy (BRD Treatment): Administer, by subcutaneous injection, a single dose of 7.5-12.5 mg/kg of body weight (3.4-5.7 mL/100 lb).
Multiple-Day Therapy (BRD Treatment): Administer daily, a subcutaneous dose of 2.5-5 mg/kg of body weight (1.1-2.3 mL/100 lb). Treatment should be repeated at 24-hour intervals for three days. Additional treatments may be given on Days 4 and 5 to animals that have shown clinical improvement but not total recovery.
Single-Dose Therapy (BRD Control): Administer, by subcutaneous injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb). Examples of conditions that may contribute to calves being at high risk for developing BRD include, but are not limited to, the following:
● Transportation with animals from two or more farm origins.
● An extended transport time with few to no rest stops.
● An environmental temperature change of ≥30°F during transportation.
● A ≥30°F range in temperature fluctuation within a 24-hour period.
● Exposure to wet or cold weather conditions.
● Excessive shrink (more than would be expected with a normal load of cattle).
● Stressful arrival processing procedures (e.g., castration or dehorning).
● Exposure within the prior 72 hours to animals showing clinical signs of BRD.
Administered dose volume should not exceed 20 mL per injection site.
Table 1 - Enroflox 100 Dose and Treatment Schedule for Cattle*
|
|
Treatment |
Control |
|
|
Weight (lb) |
Single-Dose Therapy 7.5 - 12.5 mg/kg Dose Volume (mL) |
Multiple-Day Therapy 2.5 - 5.0 mg/kg Dose Volume (mL) |
Single-Dose Therapy 7.5 mg/kg Dose Volume (mL) |
|
100 |
3.5 - 5.5 |
1.5 - 2.0 |
3.5 |
|
200 |
7.0 - 11.0 |
2.5 - 4.5 |
7.0 |
|
300 |
10.5 - 17.0 |
3.5 - 6.5 |
10.5 |
|
400 |
14.0 - 22.5 |
4.5 - 9.0 |
14.0 |
|
500 |
17.0 - 28.5 |
5.5 - 11.5 |
17.0 |
|
600 |
20.5 - 34.0 |
7.0 - 13.5 |
20.5 |
|
700 |
24.0 - 39.5 |
8.0 - 16.0 |
24.0 |
|
800 |
27.5 - 45.5 |
9.0 - 18.0 |
27.5 |
|
900 |
31.0 - 51.0 |
10.0 - 20.5 |
31.0 |
|
1000 |
34.0 - 57.0 |
11.0 - 23.0 |
34.0 |
|
1100 |
37.5 - 62.5 |
12.5 - 25.0 |
37.5 |
*Dose volumes have been rounded to the nearest 0.5 mL within the dose range.
Swine:
Administer, either by intramuscular or subcutaneous (behind the ear) injection, a single dose of 7.5 mg/kg of body weight (3.4 mL/100 lb). Administered dose volume should not exceed 5 mL per injection site.
For the control of colibacillosis, administration should be initiated within the first 60 days post-weaning when clinical signs are present in at least 2% of the animals in the group. If no improvement is noted within 48 hours, the diagnosis should be reevaluated.
Table 2 - Enroflox 100 Dose Schedule for Swine
|
Weight (lb) |
Dose Volume (mL) |
|
15 |
0.5 |
|
30 |
1.0 |
|
50 |
1.7 |
|
100 |
3.4 |
|
150 |
5.1 |
|
200 |
6.8 |
|
250 |
8.5 |
Dilution of Enroflox 100: Enroflox 100 may be diluted with sterile water prior to injection. The diluted product should be used within 24 hours. Store diluted solution in amber glass bottles between 4-40°C (36-104°F).
Table 3 - Dilution Schedule*
|
Swine Weight |
mL of Enroflox 100 |
mL of sterile water |
Number of doses |
|
10 lb |
34 mL |
66 mL |
100 |
|
15 lb |
51 mL |
49 mL |
100 |
|
20 lb |
68 mL |
32 mL |
100 |
|
25 lb |
85 mL |
15 mL |
100 |
*For 1 mL dose volume from diluted solution
100 mL vial: Use within 30 days of first puncture and puncture a maximum of 36 times. When using a needle or draw-off spike larger than 16 gauge, discard any remaining product immediately after use.
250 mL and 500 mL vials: Use within 30 days of first puncture. Puncture a maximum of 36 times with a needle or dosage delivery device 16 gauge or smaller, or 4 times with a draw-off spike 5 mm or smaller. When using a needle larger than 16 gauge, or a draw-off spike larger than 5 mm, discard any remaining product immediately after use.
 |
RESIDUE WARNINGS: Cattle: Animals intended for human consumption must not be slaughtered within 28 days from the last treatment. This product is not approved for female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. Swine: Animals intended for human consumption must not be slaughtered within 5 days of receiving a single-injection dose. |
 |
HUMAN WARNINGS:
Not for use in humans. Keep out of reach of children.
Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposures. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight. For customer service, to obtain a copy of the Safety Data Sheet (SDS) or to report adverse reactions, call Norbrook at 1-866-591-5777.
Precautions
The effects of enrofloxacin on cattle or swine reproductive performance, pregnancy and lactation have not been adequately determined.
The long-term effects on articular joint cartilage have not been determined in pigs above market weight.
Subcutaneous injection in cattle or swine, or intramuscular injection in swine, can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Enroflox 100 contains different excipients than other enrofloxacin products. The safety and efficacy of this formulation in species other than cattle and swine have not been determined.
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolone-class drugs have been shown to produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species. See Animal Safety section for additional information.
Adverse Reactions
No adverse reactions were observed during clinical trials.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Norbrook at 1-866-591-5777. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
MICROBIOLOGY:
Enrofloxacin is bactericidal and exerts its antibacterial effect by inhibiting bacterial DNA gyrase (a type II topoisomerase) thereby preventing DNA supercoiling and replication which leads to cell death.1 Enrofloxacin is active against Gram-negative and Gram-positive bacteria.
Effectiveness
Cattle: A total of 845 calves with naturally-occurring BRD were treated with enrofloxacin injection in eight field trials located in five cattle-feeding states. Response to treatment was compared to non-treated controls. Single-dose and multiple-day therapy regimens were evaluated. BRD and mortality were significantly reduced in enrofloxacin-treated calves. No adverse reactions were reported in treated animals.
The effectiveness of enrofloxacin injection for the control of respiratory disease in cattle at high risk of developing BRD was evaluated in a six-location study in the U.S. and Canada. A total of 1,150 crossbred beef calves at high risk of developing BRD were enrolled in the study. Enrofloxacin injection (7.5 mg/kg BW) or an equivalent volume of sterile saline was administered as a single subcutaneous injection within two days after arrival. Cattle were observed daily for clinical signs of BRD and were evaluated for success on Day 14 post-treatment. Treatment success in the enrofloxacin injection group (497/573, 87.83%) was significantly higher (P = 0.0013) than success in the saline control group (455/571, 80.92%). In addition, there were more treatment successes (n=13) than failures (n=3) in the group of animals positive for M. bovis on Day 0 that were treated with enrofloxacin injection. No product-related adverse reactions were reported.
Swine: A total of 590 pigs were treated with enrofloxacin injection or saline in two separate natural infection SRD field trials. For the treatment of SRD, the success rate of enrofloxacin-treated pigs that were defined as “sick and febrile” (increased respiratory rate, labored or dyspneic breathing, depressed attitude and a rectal temperature ≥104.0°F) was statistically significantly greater than the success rate of saline-treated “sick and febrile” pigs. For the control of SRD, mean rectal temperature, mortality (one trial) and morbidity were statistically significantly lower for enrofloxacin-treated pigs in pens containing a percentage of “sick and febrile” pigs compared to saline-treated pigs.
The effectiveness of enrofloxacin injection administered as a single SC dose of 7.5 mg/kg BW for the treatment and control of SRD associated with M. hyopneumoniae was demonstrated using an induced infection model study and three single-site natural infection field studies. In the model study, 72 healthy pigs were challenged with a representative M. hyopneumoniae isolate and treated with enrofloxacin injection or saline. A statistically significant (P<0.0001) decrease in the mean total lung lesion score was observed in the enrofloxacin injection-treated group (4%) compared with the saline-treated group (27%) at 10 days post-treatment. In two field studies evaluating effectiveness for treatment of SRD, a total of 300 pigs with clinical signs of SRD (moderate depression, moderately increased respiratory rate, and a rectal temperature of ≥104°F) were enrolled and treated with enrofloxacin injection or saline. At 7 days post-treatment, the cure rate was statistically significantly higher at each site (P < 0.0001) in the enrofloxacin injection-treated groups (61.3% and 92%) compared with the saline-treated groups (26.7% and 33.3%). In one field study evaluating effectiveness for control of SRD, a group of 400 pigs in which > 15% had clinical signs of SRD (moderate depression score, moderately increased respiratory rate, and a rectal temperature of ≥ 104°F) was enrolled and treated with enrofloxacin injection or saline. At 7 days post-treatment, the cure rate was statistically significantly higher (P < 0.0002) in the enrofloxacin injection-treated group (70.0%) compared with the saline-treated group (48.5%). In addition to M. hyopneumoniae, B. bronchiseptica was also isolated in sufficient numbers from these field studies to be included in the SRD treatment and control indications.
The effectiveness of enrofloxacin injection for the control of colibacillosis associated with E. coli was evaluated in a multi-site natural infection field study. At each site, when at least 5% of the pigs were defined as “clinically affected” (presence of diarrhea and either depression or gauntness), all pigs were administered enrofloxacin injection as a single IM dose of 7.5 mg/kg BW or an equivalent dose volume of saline. At 7 days post-treatment, the success rate was statistically significantly higher (P = 0.0350) in the enrofloxacin injection-treated group (61.5%) compared with the saline-treated group (44.7%).
The effectiveness of enrofloxacin injection administered as a single IM dose of 7.5 mg/kg BW for the treatment and control of SRD or as a single SC dose of 7.5 mg/kg BW for the control of colibacillosis was confirmed by demonstrating comparable serum enrofloxacin concentrations following IM or SC injection into the neck of healthy male and female pigs.
TOXICOLOGY:
The oral LD50 for laboratory rats was greater than 5000 mg/kg of body weight. Ninety-day feeding studies in dogs and rats revealed no observable adverse effects at treatment rates of 3 and 40 mg/kg respectively. Chronic studies in rats and mice revealed no observable adverse effects at 5.3 and 323 mg/kg respectively. There was no evidence of carcinogenic effect in laboratory animal models. A two-generation rat reproduction study revealed no effect with 10 mg/kg treatments. No teratogenic effects were observed in rabbits at doses of 25 mg/kg or in rats at 50 mg/kg.
ANIMAL SAFETY:
Cattle: Safety studies were conducted in feeder calves using single doses of 5, 15, and 25 mg/kg for 15 consecutive days and 50 mg/kg for 5 consecutive days. No clinical signs of toxicity were observed when a dose of 5 mg/kg was administered for 15 days. Clinical signs of depression, incoordination, and muscle fasciculation were observed in calves when doses of 15 or 25 mg/kg were administered for 10 to 15 days. Clinical signs of depression, inappetence and incoordination were observed when a dose of 50 mg/kg was administered for 3 days. No drug-related abnormalities in clinical pathology parameters were identified. No articular cartilage lesions were observed after examination of stifle joints from animals administered 25 mg/kg for 15 days.
A safety study was conducted in 23-day-old calves using doses of 5, 15, and 25 mg/kg for 15 consecutive days. No clinical signs of toxicity or changes in clinical pathology parameters were observed. No articular cartilage lesions were observed in the stifle joints at any dose level at 2 days and 9 days following 15 days of drug administration.
An injection site study conducted in feeder calves demonstrated that the formulation may induce a transient reaction in the subcutaneous tissue and underlying muscle. No painful responses to administration were observed.
Swine: Subcutaneous Safety: A safety study was conducted in 32 pigs weighing approximately 57 kg (125 lb) using single doses of 5, 15, or 25 mg/kg daily for 15 consecutive days. Incidental lameness of short duration was observed in all groups, including the saline-treated controls. Musculoskeletal stiffness was observed following the 15 and 25 mg/kg treatments with clinical signs appearing during the second week of treatment. Clinical signs of lameness improved after treatment ceased and most animals were clinically normal at necropsy.
A second study was conducted in two pigs weighing approximately 23 kg (50 lb), treated with 50 mg/kg for 5 consecutive days. There were no clinical signs of toxicity or pathological changes.
An injection site study conducted in pigs demonstrated that the formulation may induce a transient reaction in the subcutaneous tissue. No painful responses to administration were observed.
Intramuscular Safety: A safety study was conducted in 48 weaned, 20- to 22-day-old pigs. Pigs were administered enrofloxacin injection at 7.5, 22.5 and 37.5 mg/kg BW by IM injection into the neck once weekly for 3 consecutive weeks. All pigs remained clinically normal throughout the study. Transient decreases in feed and water consumption were observed after each treatment. Mild, transient, post-treatment injection site swellings were observed in pigs receiving the 37.5 mg/kg BW dose. Injection site inflammation was found on post-mortem examination in all enrofloxacin-treated groups.
STORAGE CONDITIONS:
Protect from direct sunlight. Do not refrigerate or freeze. Store below 77°F (25°C). Precipitation may occur due to cold temperature. To redissolve, warm and then shake the vial.
How Supplied
Enroflox 100:
|
100 mg/mL |
100 mL Bottle |
|
100 mg/mL |
250 mL Bottle |
|
100 mg/mL |
500 mL Bottle |
References
1. Hooper, D. C., Wolfson, J. S., Quinolone Antimicrobial Agents, 2nd ed, 59 - 75, 1993.
For customer service, to obtain a copy of the Safety Data Sheet (SDS) or to report adverse reactions, call Norbrook at 1-866-591-5777.
Restricted Drug - California. Use Only as Directed.
Made in the UK.
The Norbrook logos and Enroflox® are registered trademarks of Norbrook Laboratories Limited.
Norbrook Laboratories Limited, Newry, BT35 6PU, Co. Down, Northern Ireland
June 2021
101670I07
CPN: 1345015.6
9733 LOIRET BLVD., LENEXA, KS, 66219
| Telephone: | 913-599-5777 | |
| Fax: | 913-599-5766 | |
| Website: | www.norbrook.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
