The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Bronchi-Shield ORAL
This page contains information on Bronchi-Shield ORAL for veterinary use.The information provided typically includes the following:
- Bronchi-Shield ORAL Indications
- Warnings and cautions for Bronchi-Shield ORAL
- Direction and dosage information for Bronchi-Shield ORAL
Bronchi-Shield ORAL
This treatment applies to the following species:Bordetella Bronchiseptica Vaccine
Avirulent Live Culture
For use in Dogs Only
This product has been shown to be effective for the vaccination of healthy dogs 8 weeks of age or older against Bordetella bronchiseptica. The duration of immunity for this product has not been established. For more information regarding the efficacy and safety data go to productdata.aphis.usda.gov.
Dosage and Administration
Rehydrate with the accompanying sterile diluent. Shake well and draw back into the syringe the required amount. Remove needle from syringe. Administer 1.0 mL orally in the buccal pouch. Use immediately.The presence of maternal antibody is known to interfere with the development of active immunity in dogs and additional boosters will be required in most young animals. For advice on revaccination frequency and annual booster vaccinations, consultation with a veterinarian is recommended.
Proper oral administration
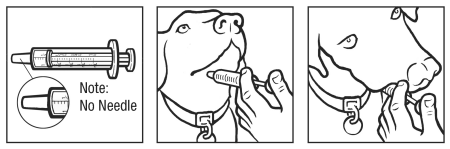
Bronchi-Shield ORAL Caution
This product is designed for oral administration. Do not vaccinate dogs parenterally. Store in the dark at 2° to 8°C (35° to 46°F). Shake well after rehydration. Inactivate all unused contents before disposal. In case of anaphylactoid reaction, administer epinephrine. Do not mix with other products, except as specified on the label. This product has not been tested in pregnant animals. In case of human exposure, contact a physician.
FOR ORAL USE ONLY. Do not vaccinate dogs parenterally.
Bronchi-Shield ORAL Caution
In the absence of a veterinarian-client-patient relationship, Federal law prohibits the relabeling, repackaging, resale, or redistribution of the individual contents of this package.
Bronchi-Shield, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2017 Elanco US Inc. all rights reserved.
Elanco US Inc., Fort Dodge, IA 50501
Phone: 888-545-5973
VLN/PCN 196/1081.02
|
25 Doses |
25 x 1 mL Vials of Vaccine plus 25 x 1 mL Vials of Diluent |
YL102605X |
CPN: 1943002.2
Distributed by ELANCO US, INC.
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
