Bravecto Quantum
This treatment applies to the following species: Company: Intervet/Merck Animal Health
Company: Intervet/Merck Animal Health
(fluralaner for extended-release injectable suspension)
150 mg fluralaner per mL when constituted
For subcutaneous use in dogs only
Bravecto Quantum Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
BRAVECTO® QUANTUM (fluralaner for extended-release injectable suspension) is an aqueous suspension consisting of two individual vials that require mixing prior to administration. One vial contains fluralaner powder and the second vial contains sterile vehicle for constitution with the powder.
Each mL of constituted drug product contains 150 mg fluralaner, 20 mg carboxymethylcellulose sodium, 20 mg benzyl alcohol, 7 mg dibasic sodium phosphate dihydrate, 1 mg poloxamer 124, and water for injection. Hydrochloric acid and sodium hydroxide are used to adjust pH.
The chemical name of fluralaner is (±) - 4 - [5 - (3,5 - dichlorophenyl) - 5 - (trifluoromethyl) - 4,5 - dihydroisoxazol - 3 - yl] - 2 - methyl - N - [2 - oxo - 2 - (2,2,2 - trifluoroethylamino)ethyl]benzamide.
Bravecto Quantum Indications
BRAVECTO QUANTUM kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and for the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick) and Rhipicephalus sanguineus (brown dog tick)] for 12 months in dogs and puppies 6 months of age and older.
BRAVECTO QUANTUM is also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for 8 months in dogs and puppies 6 months of age and older.
Dosage and Administration
Frequency of Treatment:
BRAVECTO QUANTUM should be administered as a single subcutaneous dose every 12 months.
BRAVECTO QUANTUM should be administered every 8 months in the case of potential exposure to Amblyomma americanum ticks (see Effectiveness).
Dose:
The subcutaneous dose volume is 0.1 mL of the constituted suspension/kg body weight (0.045 mL/lb). This volume provides a dose of 15 mg fluralaner/kg body weight (6.8 mg/lb). The dog should be weighed at the time of dosing to calculate an accurate dose. The following table provides a guide to weight specific dose volumes.
Guide to Body Weight Specific Dose Volumes1
|
Body Weight (lb) |
Body Weight (kg) |
Dose Volume (mL) |
|
2.2 lb |
1 kg |
0.1 mL |
|
11 lb |
5 kg |
0.5 mL |
|
22 lb |
10 kg |
1.0 mL |
|
33 lb |
15 kg |
1.5 mL |
|
44 lb |
20 kg |
2.0 mL |
|
55 lb |
25 kg |
2.5 mL |
|
66 lb |
30 kg |
3.0 mL |
|
77 lb |
35 kg |
3.5 mL |
|
88 lb |
40 kg |
4.0 mL |
|
99 lb |
45 kg |
4.5 mL |
|
110 lb |
50 kg |
5.0 mL |
|
121 lb |
55 kg |
5.5 mL |
|
132 lb |
60 kg |
6.0 mL |
1All dogs should be dosed at 0.1 mL suspension/kg body weight (0.045 mL/lb).
Treatment with BRAVECTO QUANTUM may begin at any time of the year and can continue year-round without interruption.
Constitution Procedures:
BRAVECTO QUANTUM must be prepared prior to the first use by adding the sterile vehicle to the fluralaner powder (see Constitution Procedures).
Administration Instructions:
For subcutaneous injection only. Do not administer by any other route.
1. The fluralaner powder will separate out of suspension upon standing. Before every use, shake the constituted vial vigorously for 30 seconds prior to drawing up a dose to achieve a uniform suspension.
2. Withdraw 0.1 mL of constituted suspension/kg of body weight (0.045 mL/lb) into an appropriately sized sterile syringe fitted with an 18 G needle.
3. Use another 18 G needle for dosing. Administer the dose within 2 minutes after drawing it into the dosing syringe to maintain a uniform suspension and accurate dosing. Discard if syringe sits more than 2 minutes. The dose sitting in the syringe beyond 2 minutes may decrease effectiveness.
4. Inject the product subcutaneously in the dorsoscapular region.
Contraindications
There are no known contraindications for the use of the product.
Warnings
User Safety Warnings:
Not for use in humans. Keep this and all drugs out of reach of children.
In case of accidental self-injection:
● Seek medical advice immediately and show the package insert or label to the physician.
In case of accidental skin contact:
● Wash the exposed skin with water for at least 15 minutes.
● If redness and swelling occur, seek medical advice immediately and show the package insert or label to the physician.
In case of accidental eye exposure:
● Wash the eyes with water for at least 15 minutes.
● If wearing contact lenses, rinse the eyes first, then remove contacts and continue to rinse with water.
● If redness and swelling occur, seek medical advice immediately and show the package insert or label to the physician.
Precautions
Fluralaner is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders.
BRAVECTO QUANTUM is not effective against Amblyomma americanum ticks beyond 8 months after dosing (see Effectiveness).
Prior to administration of BRAVECTO QUANTUM, owners should be informed that this product may take 3-5 days to see a notable reduction in ticks (see Effectiveness).
Hypersensitivity reactions, including anaphylaxis, have been reported with the use of this product and should be treated immediately with the same measures used to treat hypersensitivity reactions to vaccines and other injectable products (see Adverse Reactions).
The safety and effectiveness of BRAVECTO QUANTUM has not been evaluated in dogs less than 6 months of age.
The safety of BRAVECTO QUANTUM has not been evaluated in breeding, pregnant and lactating dogs. Reproductive adverse events have been reported following use of Bravecto (fluralaner) Chews in breeding females including birth defects (including limb deformities and cleft palate), stillbirth, and abortion.
Before use in breeding female dogs, refer to the Target Animal Safety section.
Adverse Reactions
In a well-controlled U.S. field study, 225 dogs were administered two doses of BRAVECTO QUANTUM at a 1-year interval and 96 dogs were administered an oral active control every 12 weeks for a total of 6 doses. Over the 455-day study period, all observations of potential adverse reactions were recorded.
Percentage of Dogs with Adverse Reactions in the Field Study
|
Adverse Reaction (AR) |
BRAVECTO QUANTUM (n = 225 dogs) |
Active Control (n = 96 dogs) |
|
Lethargy |
4.9% |
3.1% |
|
Decreased appetite |
4.4% |
4.2% |
|
Vomiting |
4.0% |
0% |
|
Diarrhea |
2.7% |
3.1% |
|
Liver enzymes (serum ALT or ALP) greater than twice the upper reference range1 |
2.7% |
2.1% |
|
Pruritus |
1.8% |
2.1% |
|
Injection site lumps or swelling2 |
1.3% |
0% |
|
Seizures3 |
0.9% |
0% |
1 Alanine aminotransferase (ALT); Alkaline phosphatase (ALP)
2 Mild injection site reactions, described as a lump, bump or knot, occurred in three BRAVECTO QUANTUM treated dogs and were all observed within 3 days of the injection. All injection site reactions were self-limiting and resolved following 1, 4, and 27 days after the initial observation.
3 Two dogs treated with BRAVECTO QUANTUM experienced seizures during the study. One dog with a history of at least one seizure within three months prior to the start of the study, reported 6 seizures in 9 months starting 23 days after the initial dose. Although anticonvulsant medications were not started, no additional seizures were observed during the study following the second dose administered 12 months after the initial dose. A second dog reported 8 seizures starting 57 days after the initial dose. The dog was removed from the study on Day 84 and managed with anticonvulsant medications.
One dog treated in the BRAVECTO QUANTUM group had a hypersensitivity reaction which included hives, facial edema, vomiting, and heavy breathing within the first 12 hours following the initial treatment. The dog was treated with oral antihistamines and recovered within 24 hours of treatment. No additional hypersensitivity reactions were observed in subsequent dosing 12 months later when premedicated with diphenhydramine.
In well-controlled foreign laboratory effectiveness studies, after administration of BRAVECTO QUANTUM, two dogs exhibited slight mucosal hyperemia the day following administration that resolved the following day, one dog exhibited transient erythema 10 minutes post-injection that resolved within 1 hour, and one dog had a nonpainful swollen upper eyelid observed 24 hours post treatment. In a large European field study, pain on injection of BRAVECTO QUANTUM was reported in 5 dogs.
Foreign Market Experience:
The following adverse events were reported voluntarily during post-approval use of BRAVECTO QUANTUM in foreign markets: gastrointestinal reactions (including vomiting, diarrhea, decreased appetite, and acute hemorrhagic diarrhea syndrome), lethargy, injection site reactions (including swelling, lump, pain, bleeding, and abscess), immune-mediated disorders (including hypersensitivity reactions, anaphylaxis, and immune-mediated hemolytic anemia), pruritus, dermatitis, and neurologic reactions (including seizures, ataxia, and tremors).
CONTACT INFORMATION:
For technical information or to report a suspected adverse event, please contact Merck Animal Health at 1-800-224-5318 or https://www.merck-animal-health-usa.com. Safety Data Sheets (SDSs) can be found at https://www.merck.com/products/safety-data-sheets/#. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or https://www.fda.gov/reportanimalae
Clinical Pharmacology
Mechanism of Action:
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
Pharmacokinetics:
The pharmacokinetics of fluralaner were evaluated in 4 male and 4 female Beagle dogs receiving a single dose of 15 mg/kg BRAVECTO QUANTUM subcutaneously. Blood samples were collected predose and 32 times post-dose for 450 days. Systemic concentrations were measurable until study termination on Day 450.
Mean (± standard deviation) Plasma Pharmacokinetic Parameters of Fluralaner
|
Parameter |
Estimate |
|
Cmax (ng/mL) |
775 ± 179 |
|
Clast (ng/mL) |
79.5 ± 47.4 |
|
Tmax1 (day) |
36 (29 - 71) |
|
AUClast (day*ng/mL) |
139000 ± 28600 |
|
AUCinf (day*ng/mL) |
158000 ± 31400 |
|
t1/2 (day) |
130 ± 27.1 |
1Median and Range
Cmax = maximum plasma concentration
Clast = plasma concentration on Day 450
Tmax = time to maximum plasma concentration
AUClast = area under the curve from the time of dosing to the last quantifiable plasma concentration
AUCinf = area under the curve from the time of dosing extrapolated to infinity
t1/2 = half-life
Following 6 doses of BRAVECTO QUANTUM administered subcutaneously every 4 months to 24 Beagle dogs at doses of 15 (1X), 45 (3X), and 75 (5X) mg/kg, AUC and Cmax were proportional over the dose range. Significant accumulation occurred with ratios of 3.9, 4.6, and 3.5 for the 1X, 3X, and 5X groups, respectively. Steady state was reached by the fourth dose (357 days) in the 1X and 3X groups and by the third dose (238 days) in the 5X group.
Effectiveness
Treatment and Prevention of Flea Infestations:
In well-controlled laboratory studies, BRAVECTO QUANTUM was 100% effective against adult fleas by 48 hours after treatment and ≥ 99.7% effective against adult fleas 24 hours post-infestation from one week through 12 months after treatment.
In a well-controlled 455-day U.S. field study conducted in households with existing flea infestations, the effectiveness of BRAVECTO QUANTUM against fleas when administered at 12-month intervals was ≥ 99.2% for 12 months. Dogs with signs of flea allergy dermatitis showed improvement in erythema, alopecia, papules, scales, crusts, and excoriation as a direct result of eliminating flea infestations.
Treatment and Control of Tick Infestations:
In a well-controlled laboratory study, the onset of effectiveness (≥ 90.0%) against preexisting infestations occurred within 3 days following post-treatment for Dermacentor variabilis and Ixodes scapularis, and within 4 days post-treatment for Rhipicephalus sanguineus. In well-controlled laboratory studies, BRAVECTO QUANTUM demonstrated ≥ 94.0% effectiveness against Rhipicephalus sanguineus ticks 48-hours post-infestation starting one week through 12 months after treatment.
In well-controlled laboratory studies, fluralaner, the active ingredient in BRAVECTO QUANTUM, demonstrated effectiveness against Ixodes scapularis and Dermacentor variabilis.
In a well-controlled laboratory study, the onset of effectiveness (≥ 90.0%) against preexisting Amblyomma americanum infestations occurred 5 days post-treatment. In well-controlled laboratory studies, BRAVECTO QUANTUM demonstrated > 93.5% effectiveness against Amblyomma americanum at 72-hours post-infestation starting approximately one week through 8 months after treatment but failed to demonstrate effectiveness beyond 8 months.
TARGET ANIMAL SAFETY:
Margin of Safety Study:
In a margin of safety study, 32 healthy intact Beagle dogs (4 dogs/sex/group) aged 6 months were administered BRAVECTO QUANTUM by subcutaneous injection at doses of 0 (0X), 15 (1X), 45 (3X), or 75 (5X) mg/kg every 4 months for a total of 6 doses (Days 1, 120, 239, 358, 477 and 596). Dogs in the control group (0X) were injected with sterile saline. Two dogs died during the study. One male in the 3X group was euthanized due to a prolapsed rectum and replaced on Day 15. One male in the 3X group was euthanized on Day 475 due to seizures. Necropsy determined this dog died of polyarteritis. The administration of BRAVECTO QUANTUM resulted in dose-volume dependent injection site swellings that resolved over time. Injection site swellings in the 1X group lasted up to 32 days after the first injection, up to 62 days after the second injection, and persisted in some dogs throughout the dosing interval after the third through sixth injections. Injection site swellings in the 3X and 5X groups occurred after each injection and persisted in some dogs throughout the dosing interval. No pain was observed during any injection site assessment. Occasional erythema occurred in all treatment groups, including dogs in the control group. Abnormal macroscopic changes at the injection sites included accumulation of tan material only in the dogs administered BRAVECTO QUANTUM. On histopathology, abnormal microscopic observation of the injection sites in dogs administered BRAVECTO QUANTUM included fibrosis (minimal to moderate), granulomatous inflammation (minimal to moderate), and/or histiocytic infiltration (minimal).
Reproductive Safety Study:
Reproductive safety was evaluated for Bravecto Chews, NADA 141-426. Bravecto Chews contains fluralaner, the same active ingredient as in BRAVECTO QUANTUM. Bravecto Chews was administered orally to intact, reproductively-sound male and female Beagle dogs at a dose of up to 168 mg/kg on three to four occasions at 8-week intervals. The dogs in the control group were untreated. There were no clinically-relevant, treatment-related effects on the body weights, food consumption, reproductive performance, semen analysis, litter data, gross necropsy (adult dogs) or histopathology findings (adult dogs and puppies).
One adult dog in the treated group suffered a seizure during the course of the study (46 days after the third treatment). Abnormal salivation was observed on 17 occasions: in six treated dogs (11 occasions) after dosing and four control dogs (6 occasions).
The following abnormalities were noted in 7 pups from 2 of the 10 dams in only the treated group during gross necropsy examination: limb deformity (4 pups), enlarged heart (2 pups), enlarged spleen (3 pups), and cleft palate (2 pups). During veterinary examination at Week 7, two pups from the control group had inguinal testicles, and two and four pups from the treated group had inguinal and cryptorchid testicles, respectively. No undescended testicles were observed at the time of necropsy (Days 50 to 71).
In a well-controlled field study BRAVECTO QUANTUM was used concurrently with other medications, such as vaccines, anthelmintics, antibiotics (including topicals), steroids, analgesics, and anesthetics. No adverse reactions were observed from the concurrent use of BRAVECTO QUANTUM with other medications.
CONSTITUTION PROCEDURES:
Items needed to constitute BRAVECTO QUANTUM
● Fluralaner powder vial - included
● Sterile vehicle vial - included
● Vent needle (25 G) - included
● Sterile transfer needle (18 G) - not included
● Sterile 20 mL syringe for transfer - not included
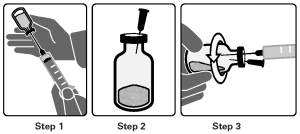
Constitution of the Product Vial:
BRAVECTO QUANTUM is supplied in 2 vials that must be combined prior to administration.
1. Shake the fluralaner powder vial to break up any aggregates prior to constitution.
2. Prior to use, invert the vehicle vial at least 3 times until visibly uniform. The vehicle solution may be clear to cloudy in appearance.
3. Using an 18 G needle and syringe, first inject up to 14 mL of air into the vehicle vial, then withdraw 15 mL of the vehicle from the vial (Step 1). There is more vehicle supplied in the vial than required for constitution.
4. Insert the 25 G vent needle into the top of the fluralaner powder vial (Step 2).
5. While rotating the vial horizontally in hand, slowly transfer the vehicle into the fluralaner powder vial to ensure complete wetting of the powder (Step 3).
6. Once the vehicle has been added, remove the vent and transfer needles from the fluralaner powder vial. Discard needles.
7. Shake the vial vigorously for at least 30 seconds until a thoroughly mixed suspension of the fluralaner powder and vehicle is produced.
8. The fluralaner powder will separate out of suspension upon standing. Before every use, shake the constituted vial vigorously for 30 seconds prior to drawing up a dose to achieve a uniform suspension.
9. Withdraw 0.1 mL of constituted suspension/kg of body weight (0.045 mL/lb) into an appropriately sized sterile syringe fitted with an 18 G needle (see Dosage and Administration).
10. Use another 18 G needle for dosing. Administer the dose within 2 minutes after drawing it into the dosing syringe to maintain a uniform suspension and accurate dosing. Discard if syringe sits more than 2 minutes. The dose sitting in the syringe beyond 2 minutes may decrease effectiveness.
11. Inject the product subcutaneously in the dorsoscapular region.
12. Discard 3 months after first puncture. Puncture a maximum of 20 times.
STORAGE CONDITIONS:
Store at or below 30°C (86°F). Discard 3 months after first puncture and puncture a maximum of 20 times.
How Supplied
BRAVECTO QUANTUM (fluralaner for extended-release injectable suspension) 20 mL vial product is available in a 1-pack presentation that includes one vial containing 2.51 grams of sterile fluralaner and one vial containing the required 15 mL of sterile vehicle for constitution.
Approved by FDA under NADA # 141-599
Formulated in Germany.
Distributed by Intervet Inc. (d/b/a Merck Animal Health), Rahway, NJ 07065.
Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Rev. 01/2025
168621 R10
CPN: 1047592.0
Intervet Inc.
126 E. LINCOLN AVENUE, PO BOX 2000, Rahway, NJ, 07065
| Customer Service: | 800-521-5767 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Website: | www.merck-animal-health-usa.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
