Atopica for Cats
This treatment applies to the following species: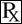 Company: Elanco US
Company: Elanco US
(cyclosporine oral solution) USP MODIFIED
100 mg/mL
Atopica for Cats Caution
Federal (USA) Law restricts this drug to use by or on the order of a licensed veterinarian.
Description
ATOPICA for Cats (cyclosporine oral solution) USP MODIFIED is an oral formulation of cyclosporine that immediately forms a microemulsion in an aqueous environment. Cyclosporine, the active ingredient in ATOPICA for Cats, is a cyclic polypeptide, immune modulating agent consisting of 11 amino acids. It is produced as a metabolite by the fungal species Beauveria nivea.
Chemically, cyclosporine A is designated designated Cyclo[[(E) - (2S,3R,4R) - 3 - hydroxy - 4 - methyl - 2 - (methylamino) - 6 - octenoyl] - L - 2 - aminobutyryl - N - methylglycyl - N - methyl - L - leucyl - L - valyl - N - methyl - L - leucyl - L - alanyl - D - ananyl - N - methyl - L - leucyl - N - methyl - L - leucyl - N - methyl - L - valyl].
The structural formula is:
Indication:
ATOPICA for Cats is indicated for the control of feline allergic dermatitis as manifested by excoriations (including facial and neck), miliary dermatitis, eosinophilic plaques, and self-induced alopecia in cats at least 6 months of age and at least 3 lbs (1.4 kg) in body weight.
Dosage and Administration
Always provide the Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats and the Information for Cat Owners with prescription.
The initial dose of ATOPICA for Cats is 3.2 mg/lb/day (7 mg/kg/day) as a single daily dose for a minimum of 4 to 6 weeks or until resolution of clinical signs. Following this initial daily treatment period, the dose of ATOPICA for Cats may be tapered by decreasing the frequency of dosing to every other day or twice weekly to maintain the desired therapeutic effect. ATOPICA for Cats should be administered directly on a small amount of food or orally just after feeding. Whenever possible, ATOPICA for Cats should be administered on a consistent schedule with regard to meals and time of day. If a dose is missed, the next dose should be administered (without doubling) as soon as possible, but dosing should be no more frequent than once daily.
The dispensing system includes an oral dosing syringe graduated in 1 lb increments. To dose the cat, the syringe should be filled to the nearest 1 lb corresponding to the cat’s body weight (round down if 0.1 to 0.4 lb, round up if 0.5 to 0.9 lb). Each pound graduation on the syringe delivers a volume of 0.032 mL providing 3.2 mg/lb. Do not rinse or clean the oral dosing syringe between uses. (See Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats)
Instructions For Assembling The Dispensing System And Preparing A Dose Of Atopica™ For Cats (cyclosporine Oral Solution) Usp Modified
Assembling The Dispensing System
The dispensing system consists of 4 parts:

1. A bottle containing the medicine, with rubber stopper and a screw cap to close the bottle after use.
2. A plastic adapter with dip tube that you will push into the neck of the bottle. The adapter must always remain in the bottle after first use.
3. An oral dosing syringe that fits into the top of the plastic adapter to withdraw the prescribed dose of medicine from the bottle.
4. A plastic vial containing the plastic adapter and oral dosing syringe. Save the plastic vial to store the oral dosing syringe between each use.
Fitting the Plastic Adapter into the New Bottle of Medicine

● Remove and save the screw cap.
● Remove and dispose of the rubber stopper.
● Hold the open bottle upright on a table and push the plastic adapter firmly into the neck of the bottle as far as you can, then close the bottle with the screw cap.
● To provide a child-resistant closure, push down on the child-resistant screw cap as you turn it.
Note: To prepare a dose, carefully follow the instructions for Preparing a Dose of Medicine.
Preparing a Dose of Medicine

1. Push and turn the child-resistant screw cap to open the bottle. Note: Always close the bottle with the child-resistant screw cap after use.
2. Check that the plunger of the oral dosing syringe is pushed all the way down.
3. Keep the bottle upright and insert the oral dosing syringe firmly into the plastic adapter.
4. Slowly pull the plunger up so that the oral dosing syringe fills with the medicine.
5. Expel any large bubbles by pushing and pulling the plunger a few times. The presence of a few tiny bubbles is not important for dosing accuracy.
6. Withdraw the dose of medicine prescribed by your veterinarian. The scale on the oral dosing syringe corresponds to the cat’s body weight.
Note: If the prescribed dose is more than the maximum volume marked on the oral dosing syringe, you will need to reload the syringe to withdraw the full dose.
7. Remove the oral dosing syringe by gently twisting it out of the plastic adapter.
You can now place the oral dosing syringe over a small amount of food or introduce the syringe in the mouth of your cat and push the medicine out of the syringe. See Information for Cat Owners for complete administration instructions.
Do not rinse or clean the oral dosing syringe between uses. Store the oral dosing syringe in the plastic tube between each use.
ATOPICA for Cats should be stored in the original container at room temperature between 59 and 77°F. Once opened, use contents within two months for the 5 mL container and 11 weeks for the 17 mL container.
Close the bottle with the child-resistant screw cap after use.
Keep out of reach of children!
Always close the bottle with the child-resistant screw cap after use. To provide a child-resistant closure, push down on the child-resistant screw cap as you turn it.
Contraindications
Do not use in cats with a history of malignant disorders or suspected malignancy.
Do not use in cats infected with feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV).
Do not use in cats with a hypersensitivity to cyclosporine.
Warnings
ATOPICA for Cats is a systemic immunosuppressant that may increase the susceptibility to infection and the development of neoplasia. One of 205 field study cats died of the effusive form of feline infectious peritonitis. (See Adverse Reactions)
Persistent, progressive weight loss that resulted in hepatic lipidosis occurred in 2 of 205 cats on treatment with ATOPICA for Cats in field studies. Monitoring of body weight is recommended. (See Adverse Reactions)
Human Warnings:
Not for human use. Keep this and all drugs out of reach of children. For use only in cats.
Special precautions to be taken when administering ATOPICA for Cats:
Do not eat, drink, smoke, or use smokeless tobacco while handling ATOPICA for Cats.
Wash hands after administration.
In case of accidental ingestion, seek medical advice immediately and provide the package insert or the label to the physician.
People with known hypersensitivity to cyclosporine should avoid contact with ATOPICA for Cats.
Precautions
The safety and effectiveness of ATOPICA for Cats has not been established in cats less than 6 months of age or less than 3 lbs (1.4 kg) body weight.
ATOPICA for Cats is not for use in breeding cats, pregnant or lactating queens.
Cats should be tested and found to be negative for FeLV and FIV infections before treatment.
As with any immunosuppressive regimen, exacerbation of sub-clinical neoplastic and infectious conditions may occur. ATOPICA for Cats is not for use with other immunosuppressive agents.
Cats that are seronegative for Toxoplasma gondii may be at risk of developing clinical toxoplasmosis if they become infected while under treatment, which can be fatal. In a controlled laboratory study, cats seronegative for T. gondii were administered cyclosporine and subsequently infected with T. gondii, resulting in increased susceptibility to infection and subsequent expression of toxoplasmosis.
Cyclosporine did not increase T. gondii oocyst shedding (see Animal Safety). Potential exposure of seronegative cats to T. gondii should be avoided (e.g. keep indoors, avoid raw meat or scavenging).
In cases of clinical toxoplasmosis or other serious systemic illness, stop treatment with cyclosporine and initiate appropriate therapy.
ATOPICA for Cats may cause elevated levels of serum glucose, creatinine, and urea nitrogen. ATOPICA for Cats should be used with caution in cases with diabetes mellitus or renal insufficiency.
ATOPICA for Cats should be used with caution with drugs that affect the P-450 enzyme system. Simultaneous administration of ATOPICA for Cats with drugs that suppress the P-450 enzyme system, such as azoles (e.g. ketoconazole), may lead to increased plasma levels of cyclosporine.
Treatment with ATOPICA for Cats may result in decreased immune response to vaccination. Naïve cats may not develop protective titers during treatment (see Animal Safety).
Adverse Reactions
The clinical safety of ATOPICA for Cats was assessed in a masked, controlled 6-week field study followed by a 12-week open-labeled dose-tapering field study. In these two field studies, 205 cats received treatment with ATOPICA for Cats for up to 126 days.
Two cats died or were euthanized within two weeks following study exit. One cat was diagnosed with the effusive form of feline infectious peritonitis and died following normal study exit, and one cat with pre-existing anemia that worsened during the study was diagnosed with aplastic anemia and euthanized because of a poor prognosis for recovery.
Fourteen of the 205 cats (6.8%) were withdrawn from the studies due to the occurrence of an adverse reaction. Adverse reactions in these 14 cats included weight loss, anorexia, vomiting, diarrhea, hypersalivation, lethargy, hepatic lipidosis and jaundice, upper respiratory signs, ocular discharge, cough, toxoplasmosis, lymphopenia, anemia, bacterial dermatitis, seizure, ataxia, and small cell gastrointestinal lymphoma.
The most commonly reported adverse reaction was vomiting. In most cases, vomiting spontaneously resolved with continued dosing. Adverse reactions occurred most often with daily dosing compared to other dosing regimes.
Adverse reactions reported with greater than 2% frequency in the two field studies.
|
Adverse Reaction* |
Number (Percent) of Cases n = 205 |
|
Vomiting/Retching/Regurgitation |
72 (35.1%) |
|
Weight Loss |
42 (20.5%) |
|
Diarrhea |
31 (15.1%) |
|
Anorexia/Decreased Appetite |
29 (14.1%) |
|
Lethargy/Malaise |
28 (13.6%) |
|
Hypersalivation |
23 (11.2%) |
|
Behavioral Disorder (hiding, hyperactivity, aggression) |
18 (8.8%) |
|
Ocular Discharge/Epiphora/Conjunctivitis |
14 (6.8%) |
|
Sneezing/Rhinitis |
11 (5.4%) |
|
Gingivitis/Gingival Hyperplasia |
9 (4.4%) |
|
Polydipsia |
6 (2.9%) |
*Cats may have experienced more than one type or occurrence of a reaction during the studies.
The following adverse reactions were reported in less than or equal to 2% of cats treated with ATOPICA for Cats in the two field studies: bacterial dermatitis, hepatic lipidosis and jaundice, gastrointestinal small cell lymphoma, constipation, cough, toxoplasmosis, muscle wasting, muscle tremors, ataxia, convulsion, polyuria, urinary tract infection, inappropriate urination or defection, seborrhea, worsening otitis externa, papilloma, leukotrichia (whitening of hair) and excessive hair growth, anemia, lymphopenia, worsening monocytosis, worsening neutrophilia, hyperglobulinemia, increased serum creatinine and urea nitrogen, and increased alanine aminotransferase.
To report adverse effects, access medical information, or obtain additional product information call 1-888-545-5973. Alternatively, suspected adverse drug reactions may be reported to FDA at 1-800-FDA-VETS or http://www.fda.gov/reportanimalae
Information for Cat Owners:
Owners should be advised to discontinue ATOPICA for Cats and contact their veterinarian in case of signs of serious illness and/or persistent, progressive weight loss. Owners should be informed of the risks of increased susceptibility to infection and the development of neoplasia, and they should be provided advice on how to avoid exposure of their cat to Toxoplasma gondii infection.
Clinical Pharmacology
Cyclosporine is an immunosuppressive agent that has been shown to work via suppression of T-helper and T-suppressor cells and inhibition of interleukin-2. It does not depress hematopoesis or the function of phagocytic cells. ATOPICA for Cats is not a corticosteroid or antihistamine.
Following an intravenous dose of 2 mg/kg in a 24-hour fasted state, clearance of cyclosporine A in cats was 0.199 L/kg x h and half life was ~24 hours. After oral administration, the terminal elimination half life has been estimated to be as short as 6.8 to longer than 40 hours in some normal healthy cats.
The bioavailability of ATOPICA for Cats is highly variable both within and between cats. A pharmacokinetic study showed no consistent difference in the mean extent of drug absorption when administered orally to fed or fasted cats or mixed in with food.
Blood levels of cyclosporine in field studies were highly variable, even among cats with similar clinical response, suggesting no generalizable correlations can be made between cats with regard to blood cyclosporine levels and clinical response (effectiveness and safety). Nevertheless, individual differences in the relationship between drug exposure and clinical response may exist. Therefore, to minimize individual fluctuations in drug absorption, ATOPICA for Cats should be administered on a consistent schedule with regard to meals and time of day.
Effectiveness
A masked, controlled field study was conducted at 24 sites from various geographic locations in the United States and Canada. In this study, 217 client-owned cats with clinical signs consistent with allergic dermatitis (miliary dermatitis, excoriations including facial or neck, self-induced alopecia and eosinophilic plaques) along with non-seasonal localized or generalized pruritus, were randomly assigned in a 2:1 ratio and received either ATOPICA for Cats or a control solution (the excipients of ATOPICA for Cats without the cyclosporine). Owners administered treatment in a small amount of food or directly in the cat’s mouth just after feeding once daily for up to 6 weeks. No additional therapy with antihistamines, corticosteroids or medicated shampoos was permitted.
Effectiveness was evaluated in 181 cats. Cats in the ATOPICA for Cats treatment group had a 65.1% reduction in mean total lesion score, compared to cats in the control treatment group, which had a 9.2% reduction in mean total lesion score. The percent of cats identified as treatment success by the Owner was 78.6% in the ATOPICA for Cats group compared to 26.2% in the control group. Compared to the control group, the ATOPICA for Cats group had improved mean ratings for Investigator assessment of overall improvement, Owner and Investigator assessment of pruritus, and number of body regions with lesions.
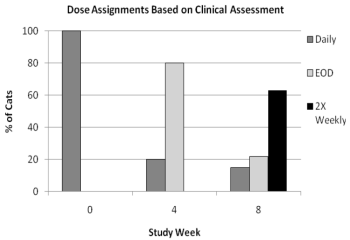
After drop-out from or completion of the masked 6-week field study, 191 cats were enrolled in a 12-week open-labeled field study to evaluate dose tapering of ATOPICA for Cats. The graph below shows the dose assignments for each 4-week dosing period. At study entry, all cats were assigned daily doses. At Week 4, cats were assigned daily or every other day (EOD) dosing, based on clinical improvement. At Week 8, cats were assigned daily, EOD, or twice weekly (2X Weekly) dosing for the final month of the study. Cats with poor responses exited the study at Weeks 4 and 8. At study exit at Week 12, 62.9%, 21.6%, and 15.5% of the remaining 97 evaluable cats were on twice weekly, EOD, and daily dosage regimens, respectively.
ATOPICA for Cats was used in conjunction with various medications including a macrocyclic lactone and other antiparasitic agents, systemic antimicrobials, and topical skin and otic cleansers and antimicrobials.
Animal Safety:
In a 6-month safety study, forty (20 male and 20 female) 6-month old cats were randomized into 5 treatment groups and administered 0, 8, 16, 24 or 40 mg/kg/day ATOPICA for Cats (0, 1, 2, 3 or 5X the maximum therapeutic dose). An intermittent interventricular conduction disturbance was noted on electrocardiogram in one 3X and one 5X treatment group cat following 6 months of dosing. A 5X cat was euthanized after two weeks of treatment following a rapidly-declining clinical condition including recumbency, inappetance, dehydration, and decreased body weight. A post-mortem examination showed a healing rib fracture and bone marrow hypocellularity characterized by a moderate reduction in the number of bone marrow cells from multiple lineages. Hematology parameters drawn prior to euthanasia for this cat did not reveal abnormalities indicative of bone marrow hypocellularity. A 5X female cat presented with abdominal fibroadenomatous nodules during the study. Lymphoma of the kidneys and a mesenteric lymph node were present on necropsy in one 5X male, which is likely related to the immunosuppressive effects of cyclosporine treatment. Activated partial thromboplastin time (APTT) was prolonged in treated cats when compared to control cats.
A safety study was conducted to evaluate the effect of ATOPICA for Cats on the development of vaccine titers following vaccination in cats. Thirty-two cats (16 males and 16 females) were randomized into two treatment groups. Group 1 cats served as the control group and were sham dosed. Group 2 cats were administered ATOPICA for Cats at a dose of 24 mg/kg (3X the maximum therapeutic dose) orally once daily for 56 days. All cats were approximately 7 months of age at the start of the study and previously vaccinated against feline calici virus (FCV), feline panleukopenia virus (FPV), feline leukemia virus (FeLV), feline herpes virus-1 (FHV-1) and rabies with the final pre-treatment vaccines administered 16 weeks prior to treatment with cyclosporine. Cats were naïve to the feline immunodeficiency virus (FIV) vaccine, which was administered after 28 days on cyclosporine. After booster vaccinations on Day 28, titers for FCV, FPV, FeLV, FHV-1 and rabies were decreased in cyclosporine treated cats compared to control cats, but these titers remained adequate in both treatment groups. In contrast, cats on high-dose cyclosporine failed to develop titers to the novel vaccine (FIV). An increase in incidence and frequency of diarrhea, vomiting, and salivation were noted in Group 2 cats. One female cat treated with cyclosporine was observed to be in estrus during the study compared to 5 of the female control cats. One cat treated with cyclosporine was noted as having a slow or absent startle reflex, displayed ataxia, had small lymph nodes, thin body condition, and gas and fluid filled loops of intestine. Lymphocyte counts were lower in treated cats when compared to control. APTT was prolonged in treated cats when compared to control cats. Cholesterol, glucose, total protein, blood urea nitrogen, and creatinine values were elevated in cyclosporine treated cats with values just above the normal reference range. Glucosuria was noted in three treated animals that also had hyperglycemia.
A safety study was conducted to evaluate the effects of ATOPICA for Cats on the clinical course of Toxoplasma gondii. Thirty domestic short-haired cats (15 males and 15 females) ranging in age from 1-2 years were randomized into three treatment groups. Group 1 cats served as the control group and were administered placebo. Group 2 cats were administered placebo for 84 days followed by treatment with ATOPICA for Cats for 42 days. Group 3 cats were treated with ATOPICA for Cats for 126 days. ATOPICA for Cats was administered at a target dose of 7.5 mg/kg orally once daily. All cats were infected with T. gondii cysts on Study Day 42. One cat was found dead and another was euthanized (both in Group 3) within six weeks following infection due to complications related to toxoplasmosis. Clinical signs typical of T. gondii infection, including bloody feces, lethargy, and vomiting/regurgitation, were also seen in most of the remaining cats, but resolved within six weeks following infection. Decreases in body weight and food consumption were seen in some cats from each group, but these changes were reversible as the animals recovered from clinical toxoplasmosis. APTT was prolonged in Group 2 and 3 cats receiving cyclosporine when compared to Group 1 cats. Cholesterol, glucose and total protein/globulin values were elevated in cyclosporine treated cats. Ocular changes consistent with toxoplasmosis were seen in one to two cats in each group. The oocyst shedding period and number of oocysts shed were increased in Group 1 and 2 cats compared to Group 3 cats. All inoculated cats developed T. gondii IgG antibodies; IgM titers were detected in only 3 cats. Post-mortem examinations revealed mild to moderate inflammation in the central nervous system and pulmonary tissues, with the highest incidence and severity generally following this trend: Group 3 > Group 2 > Group 1. Lesions were consistent with T. gondii infection and were more prevalent in males than females. T. gondii organisms were only detected histopathologically in the tissues of the two Group 3 cats that died of toxoplasmosis.
Storage Information:
ATOPICA for Cats should only be dispensed in the original container and stored at controlled room temperature between 59 and 77°F (15-25°C). Once opened, use contents within two months for the 5 mL container and 11 weeks for the 17 mL container.
How Supplied
ATOPICA for Cats (cyclosporine oral solution) USP MODIFIED is supplied in glass amber bottles of 5 and 17 mL at 100 mg/mL. A dispensing system is included (See Instructions for Assembling the Dispensing System and Preparing a Dose of ATOPICA for Cats).
Manufactured for:
Elanco US Inc. Greenfield, IN 46140, USA
Product of Ireland
Approved by FDA under NADA # 141-329
Revision Date: August 2021
Atopica, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2021 Elanco or its affiliates
PA231163X
W1b
CPN: 1131030.5
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
