Halothane: Package Insert / Prescribing Info
Package insert / product label
Dosage form: inhalant
Drug class: General anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 24, 2025.
On This Page
Halothane Description
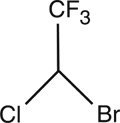
Halothane, USP is an inhalation anesthetic chemically designated 2-bromo-2-chloro-1,1,1-trifluoroethane. The specific gravity is 1.872 to 1.877 at 20° C, and the boiling point (range) is 49° to 51° C, at 760 mm Hg. The vapor pressure is 243 mm Hg at 20° C. The blood/gas coefficient is 2.5 at 37° C, and the olive oil/water coefficient is 220 at 37° C. Vapor concentrations within anesthetic range are nonirritating and have a pleasant odor. Halothane is nonflammable, and its vapors mixed with oxygen in proportions from 0.5 to 50% (v/v) are not explosive. Halothane has a molecular weight of 197.38 and a chemical formula of C2HBrClF3.
Halothane does not decompose in contact with warm soda lime. When moisture is present, the vapor attacks aluminum, brass and lead, but not copper. Rubber, some plastics, and similar materials are soluble in Halothane; such materials will deteriorate rapidly in contact with Halothane vapor or liquid. Stability of liquid Halothane is maintained by the addition of 0.01% thymol (w/w), and storage is in amber colored bottles.
Halothane should not be kept indefinitely in vaporizer bottles not specifically designed for its use. It is recommended that vaporizers be emptied at the end of each operating day. Thymol, which does not volatilize along with Halothane, accumulates in the vaporizer, and may, in time, impart a yellow color to the remaining liquid or to wicks in vaporizers. The development of such discoloration may be used as an indicator that the vaporizer should be drained and cleaned, and the discolored Halothane discarded. Accumulation of thymol may be removed by washing with diethyl ether. After cleaning a wick or vaporizer, make certain all the diethyl ether has been removed before reusing the equipment to avoid introducing ether into the system.
ACTIONS
Volatilized Halothane, USP acts as an inhalation anesthetic. Induction and recovery are rapid and depth of anesthesia can be rapidly altered. Halothane anesthesia progressively depresses respiration. There may be tachypnea with reduced tidal volume and alveolar ventilation. Halothane vapor is not an irritant to the respiratory tract, and no increase in salivary or bronchial secretions ordinarily occurs. Pharyngeal and laryngeal reflexes are rapidly obtunded. It causes bronchodilation. Hypoxia, acidosis, or apnea may develop during deep anesthesia.
Halothane anesthesia reduces the blood pressure, and frequently decreases the pulse rate. The greater the concentration of the drug, the more evident these changes become. Atropine may reverse the bradycardia. Halothane anesthesia also causes dilation of the vessels of the skin and skeletal muscles. It does not cause the release of catecholamines from adrenergic stores.
Cardiac arrhythmias may occur during Halothane anesthesia. These include nodal rhythm, A-V dissociation, ventricular extra systoles and asystole. Halothane sensitizes the myocardial conduction system to the action of epinephrine and levarterenol (norepinephrine), and the combination may cause serious cardiac arrhythmias. Halothane anesthesia increases cerebral spinal fluid pressure and produces moderate muscular relaxation. Muscle relaxants are used as adjuncts in order to maintain lighter levels of anesthesia. Halothane anesthesia augments the action of nondepolarizing skeletal muscle relaxants and ganglionic blocking agents. Halothane also is a potent uterine relaxant.
Indications and Usage for Halothane
Halothane is indicated for the induction and maintenance of general anesthesia.
Contraindications
Halothane is not recommended for obstetrical anesthesia except when uterine relaxation is required.
Warnings
When previous exposure to Halothane was followed by unexplained jaundice, consideration should be given to the use of other agents.
Halothane, USP should be used in vaporizers that permit a reasonable approximation of output, and preferably of the calibrated type. The vaporizer should be placed out of circuit in closed circuit rebreathing systems; otherwise overdosage is difficult to avoid. The patient should be closely observed for signs of overdosage, i.e., depression of blood pressure, pulse rate and ventilation, particularly during assisted or controlled ventilation.
Usage in Pregnancy.
Safe use of Halothane has not been established with respect to possible adverse effects upon fetal development. Therefore, Halothane should not be used in women where pregnancy is possible and particularly during early pregnancy, unless, in the judgment of the physician, the potential benefits outweigh the unknown hazards to the fetus.
Precautions
The uterine relaxation obtained with Halothane, unless carefully controlled, may fail to respond to ergot derivatives and oxytocic posterior pituitary extract (oxytocin injection).
Halothane increases cerebrospinal fluid pressure. Therefore, in patients with markedly raised intracranial pressure, if Halothane is indicated, administration should be preceded by measures ordinarily used to reduce cerebrospinal fluid pressure. Ventilation should be carefully assessed, and it may be necessary to assist or control ventilation to insure adequate oxygenation and carbon dioxide removal.
Epinephrine or levarterenol (norepinephrine) should be employed cautiously, if at all, during Halothane anesthesia since their simultaneous use may induce ventricular tachycardia or fibrillation.
Nondepolarizing relaxants and ganglionic blocking agents should be administered cautiously, since their actions are augmented by Halothane.
It has been reported that in genetically susceptible individuals, the use of general anesthetics and the muscle relaxant, succinylcholine, may trigger a syndrome known as malignant hyperthermic crisis. Monitoring temperature during surgery will aid in early recognition of this syndrome. Dantrolene sodium and supportive measures are generally indicated in the management of malignant hyperthermia. Consult literature references or the dantrolene prescribing information for additional information about the management of malignant hyperthermic crisis.
Adverse Reactions/Side Effects
The following adverse reactions have been reported: Hepatic necrosis, cardiac arrest, hypotension, respiratory arrest, cardiac arrhythmias, hyperpyrexia, shivering, nausea and emesis.
Related/similar drugs
Halothane Dosage and Administration
Halothane, USP may be administered by the nonrebreathing technique, partial rebreathing, or closed technique. The induction dose varies from patient to patient. The maintenance dose varies from 0.5 to 1.5%.
Halothane may be administered with either oxygen or a mixture of oxygen and nitrous oxide.
How is Halothane supplied
Halothane, USP (List No. 4894), is supplied in amber colored 250 mL glass bottles, stabilized with thymol 0.01% (w/w).
Store in cool, dry place and protect from undue exposure to light.
June, 2005
| ©Hospira 2005 | EN-0953 | Printed in USA |
| Marketed by HOSPIRA, INC., LAKE FOREST, IL 60045 USA |
||
| HALOTHANE
halothane inhalant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. |