Primaquine: Package Insert / Prescribing Info
Package insert / product label
Generic name: primaquine phosphate
Dosage form: tablet, film coated
Drug class: Antimalarial quinolines
Medically reviewed by Drugs.com. Last updated on Apr 22, 2025.
On This Page
Primaquine Description
Primaquine phosphate is 8-[(4-amino-1-methylbutyl) amino]-6-methoxyquinoline phosphate, a synthetic compound with potent antimalarial activity. The molecular formula of Primaquine phosphate is C15H21N3O·2H3PO4 and its molecular weight is 455.34. The structural formula of Primaquine phosphate is:
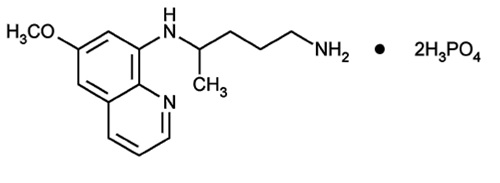
Figure 1: Primaquine phosphate structure.
Each Primaquine phosphate tablet contains 26.3 mg of primaquine phosphate (equivalent to 15 mg of primaquine base). The dosage is customarily expressed in terms of the base.
Inactive Ingredients: Microcrystalline Cellulose, Pregelatinized Starch, Lactose Monohydrate, Magnesium Stearate, Purified Water, Hypromellose, Opadry Purple, Titanium Dioxide, Macrgol/PEG, FD&C Red #40 and FD&C Blue #2.
Primaquine - Clinical Pharmacology
Mechanism of Action
Primaquine phosphate is an 8-aminoquinoline antimalarial drug. The mechanism of action has not been fully established. The major assumptions are an inhibition of the mitochondrial system of dormant parasites, and an oxidative stress generated through reactive metabolites in infected cells. In humans, primaquine phosphate activity is probably related to hydroxylated metabolites generated intrahepatically by CYP2D6.
Antimicrobial Activity
Primaquine phosphate is active against the dormant liver forms of P.vivax, namely hypnozoites, as well as exoerythrocytic stages of the parasite. Thereby, it prevents the development of the blood (erythrocytic) forms of the parasite which are responsible for relapses in vivax malaria. Primaquine phosphate is also active against gametocytes of Plasmodium falciparum.
Resistance
Development of resistance to primaquine phosphate in Plasmodium species has not been well studied.
Pharmacokinetics
Following single oral dosing, the Cmax and AUC of primaquine increase approximately dose-proportionally over a primaquine base dose range of 15 mg to 45 mg (3 times the approved dose).
The pharmacokinetic parameters and properties of primaquine and carboxyprimaquine (main circulating metabolite not expected to be active) in patients with P. vivax malaria following Oral Administration of Primaquine phosphate Tablets are provided in TABLE 1.
| PK Parametera
| Day
| Primaquine
| Carboxyprimaquine
|
||
| Cmax (ng/mL) | 1 | 50.7 ± 21.2 | 291 ± 52 |
||
| Cmax (ng/mL) | 14 | 49.7 ± 14.4 | 432 ±112 |
||
| AUC or AUC0-24 (μg/mL*h)b
| 1 | 0.48 ± 0.26 | 5.15 ± 1.01 |
||
| AUC or AUC0-24 (μg/mL*h)b
| 14 | 0.49 ± 0.19 | 7.24 ± 1.82 |
||
| Primaquine
|
|||||
| Absorption
|
|||||
| Bioavailabilityc
| >70 % |
||||
| Tmax
| 2.3 ± 1.1 hours |
||||
| Effect of food on Primaquine Phosphate Tablet (relative to fasting)d Geometric mean [95% confidence interval] | ↑ 14% [3, 27] (AUC); ↑ 26% [12, 40] (Cmax) |
||||
| Distribution
|
|||||
| % Bound to human plasma proteins | 74% (mainly to alpha 1 acid glycoprotein) |
||||
| Volume of distribution (V)e
| 243 ± 69 L |
||||
| Metabolism
|
|||||
| Metabolic pathways | -Oxidative deamination, MAO-A -Hydroxylation of the quinoline ring, CYP2D6 -Direct conjugations |
||||
| Elimination
|
|||||
| Major route of elimination | Metabolism |
||||
| Apparent Clearance (CL/F) | 37.6 ± 14.7 L/hr |
||||
| Mean terminal half-life (t1/2)f
| 5.6 ± 1.0 hours |
||||
| % of dose excreted in urine g, h
| 64%, (including 3.6% of primaquine, the remnant being metabolites other than carboxyprimaquine) |
||||
| Cmax=maximum plasma concentration; AUC=area under the plasma concentration-time curve from time zero up to infinity; MAO-A = monoamine oxidase A |
|||||
| a 15 mg once daily in adult patients (18 years of age and older) with P. vivax malaria, unless otherwise specified b AUC for primaquine, AUC0-24 for carboxyprimaquine c Healthy participants d Values refer to increase in mean systemic exposure with bread and butter: 82% fat, ~28g fat after single dose of 30 mg Primaquine in healthy participants e IV dose administration of [14C]-primaquine in healthy participants f The mean terminal half-life of carboxyprimaquine is approximately 22 hours g Oral administration of [14C]-primaquine in healthy participants; no data in feces h The main circulating metabolite, carboxyprimaquine is subjected to further metabolism and not eliminated through urine |
|||||
Gender and ethnicity
No gender nor ethnicity effect has been evidenced in studies conducted to date.
Elderly patients
There are no pharmacokinetics studies in patients older than 52 years of age.
Hepatic impairment
Single dose pharmacokinetics study performed in patients with mild or moderate hepatic impairment indicate that only moderate hepatic dysfunction impacted significantly the PK of primaquine with a 3-fold lower primaquine Cmax in patients with moderate hepatic dysfunction as compared to healthy subjects. The primaquine AUC was not significantly modified.
No data are available after repeated dosing in patients with hepatic impairment. It is not known whether in patients with hepatic impairment, accumulation of primaquine and its metabolites could occur or if there could be an impact on generation of metabolites contributing to pharmacological activity.
Renal impairment
Single dose pharmacokinetics studies performed in patients with chronic severe (eGFR 15 to 29 mL/min) or end-stage (< 15 mL/min) renal impairment indicate higher primaquine Cmax (up to 1.7-fold higher as compared to healthy subjects) but no evidence of major difference in AUC or t1/2. It is not known whether after repeated dosing there could be an accumulation of metabolites that are mainly excreted by renal route.
Drug Interaction Studies
Effect of other Drugs on the Pharmacokinetics of primaquine
In vitro data suggest primaquine is not a substrate of either P-gp or BCRP membrane transporters.
Effect of primaquine on the Pharmacokinetics of other drugs
In vitro data suggest primaquine has the potential to inhibit CYP1A2 enzyme activity, but no or low potential to inhibit MAO-A, MAO-B, or CYP450 isoforms 2A6, 2C8, 2C9, 2C19, 2D6, 3A4 enzymes involved in drug biotransformation.
In vitro data suggest primaquine has the potential to inhibit the P-gp membrane transporter.
Pharmacogenomics
Based on experiments in mice, primaquine activity probably depends on the formation of CYP2D6 metabolite(s). CYP2D6 polymorphism may be associated with variability in clinical response to Primaquine phosphate Tablets.
Indications and Usage for Primaquine
Primaquine phosphate Tablets are indicated for the radical cure (prevention of relapse) of vivax malaria.
Contraindications
Known hypersensitivity reactions to primaquine phosphate, other 8-aminoquinolones, or to any component in Primaquine phosphate Tablets.
Severe glucose-6-phosphate dehydrogenase (G6PD) deficiency (see WARNINGS, Hemolytic Anemia).
Pregnant women (see WARNINGS, Pregnancy).
Breastfeeding by a lactating woman when the infant is found to be G6PD deficient or if G6PD status is unknown (see WARNINGS, Nursing Mothers).
Because quinacrine hydrochloride appears to potentiate the toxicity of antimalarial compounds which are structurally related to primaquine, the use of quinacrine in patients receiving Primaquine phosphate Tablets is contraindicated. Similarly, Primaquine phosphate Tablets should not be administered to patients who have received quinacrine recently, as toxicity is increased.
Warnings
Hemolytic Anemia
Hemolytic reactions (moderate to severe) may occur in individuals with G6PD deficiency and in individuals with a family or personal history of favism. Areas of high prevalence of G6PD deficiency are Africa, Southern Europe, Mediterranean region, Middle East, South-East Asia, and Oceania. People from these regions have a greater tendency to develop hemolytic anemia due to a congenital deficiency of erythrocytic G6PD while receiving primaquine and related drugs.
Due to the risk of hemolytic anemia in patients with G6PD deficiency, G6PD testing must be performed before using primaquine. Before initiating treatment, obtain baseline hemoglobin and hematocrit. In case of severe anemia, postpone the G6PD test and decision on treatment with primaquine until recovery.
Due to the limitations of G6PD tests, physicians need to be aware of residual risk of hemolysis and adequate medical support and follow-up to manage hemolytic risk should be available. This is of particular importance in individuals with a personal or family history of hemolytic anemia.
Patients with G6PD Deficiency
Primaquine phosphate Tablets are contraindicated in patients with severe G6PD deficiency (see CONTRAINDICATIONS).
In case of mild to moderate G6PD deficiency, a decision to prescribe primaquine must be based on an assessment of the risks and benefits of using primaquine. If primaquine administration is considered, baseline hematocrit and hemoglobin must be checked before treatment and close hematological monitoring (e.g., at day 3 and 8) is required. Adequate medical support to manage hemolytic risk should be available.
Patients with Unknown G6PD Status
When the G6PD status is unknown and G6PD testing is not available, a decision to prescribe primaquine must be based on an assessment of the risks and benefits of using primaquine. Risk factors for G6PD deficiency or favism must be assessed. Baseline hematocrit and hemoglobin must be checked before treatment and close hematological monitoring (e.g., at day 3 and 8) is required. Adequate medical support to manage hemolytic risk should be available.
Patients without G6PD Deficiency
In G6PD normal patients it is also advisable to perform routine blood examinations (particularly blood cell counts and hemoglobin determinations) during therapy.
Risk of Hemolysis with Other Drugs
Avoid the concurrent administration of hemolytic agents in all patients (see CLINICAL PHARMACOLOGY, Drug Interactions). Warn patients to discontinue the use of Primaquine phosphate Tablets promptly if signs suggestive of hemolytic anemia occur (such as darkening of the urine, pale skin, shortness of breath, dizziness, and fatigue) and to contact their healthcare professional immediately.
Pregnancy
Safe usage of Primaquine phosphate Tablets in pregnancy has not been established. Primaquine is contraindicated in pregnant women. The use of Primaquine phosphate Tablets during pregnancy may cause hemolytic anemia in a G6PD-deficient fetus. Even if a pregnant woman has normal levels of G6PD, the fetus could be G6PD-deficient (see CONTRAINDICATIONS). Animal data show toxicity to reproduction and embryofetal development. (See PRECAUTIONS, Animal Pharmacology and/or Animal Toxicology).
Nonclinical data from studies conducted in bacteria and in animals treated with primaquine show evidence of gene mutations and chromosomal/DNA damage, teratogenicity, and injury to embryos and developing fetuses when primaquine is administered to pregnant animals. Inform patients of the potential for adverse genetic and reproductive effects associated with primaquine treatment (see PRECAUTIONS, Carcinogenesis, Mutagenesis, and Impairment of Fertility, and Animal Pharmacology and/or Animal Toxicology).
Use in Females and Males of Reproductive Potential
Pregnancy Testing
Sexually active females of reproductive potential should have a pregnancy test prior to starting treatment with primaquine.
Contraception
Patients should avoid pregnancy during treatment. The use of effective contraception is recommended during treatment and after the end of treatment as follows: Advise sexually active females of childbearing potential to use effective contraception (methods that result in less than 1% pregnancy rates) when using primaquine and after stopping treatment until 2 menses have elapsed). Advise treated males whose partners may become pregnant, to use a condom while on treatment and for 3 months after stopping treatment with primaquine.
Nursing Mother
A breastfed infant with G6PD deficiency is at risk for hemolytic anemia from exposure to primaquine. Infant G6PD status should be checked before breastfeeding begins. Primaquine phosphate Tablets are contraindicated in breastfeeding women when the infant is found to be G6PD deficient or the G6PD status of the infant is unknown (see CONTRAINDICATIONS). Advise the woman with a G6PD-deficient infant or if the G6PD status of the infant is unknown not to breastfeed.
The presence of primaquine and its major metabolite in breast milk and infant plasma were evaluated in a published study of 21 G6PD-normal lactating women and their G6PD-normal infants aged 28 days or older. After repeat administration of a 0.5 mg/kg/day primaquine base dose for 14 days in the lactating women, low concentrations of primaquine and carboxyprimaquine were measured both in breast milk and in infant plasma. The estimated infant ingested dose was found to be less than 1% of a 0.5 mg/kg/day primaquine base dose determined from an observed milk to maternal plasma AUC ratio of 0.34 (range: 0.12 to 0.64) and assuming an infant milk consumption of 150 mL/kg/day. Infant primaquine concentrations in plasma were below measurement thresholds (2.28 ng/mL) in all but 1 infant capillary plasma sample (2.6 ng/mL), and carboxyprimaquine concentrations in plasma were likewise unmeasurable in the majority of infant samples (range, 4.88 ng/mL [measurement threshold] to maximum value 25.8 ng/mL). There is no information on the effects of Primaquine phosphate Tablets on the breastfed infant, or the effects on milk production.
Precautions
Methemoglobinemia
Primaquine may cause a transient increase in methemoglobin levels up to 10% in patients without risk factors (see ADVERSE REACTIONS). Methemoglobinemia may be severe in patients who are deficient in nicotinamide adenine dinucleotide (NADH), methemoglobin reductase or treated with methemoglobinemia-inducing drugs such as dapsone or sulfonamide, (see PRECAUTIONS, Drug Interactions). Monitor methemoglobin levels closely in these cases.
Advise all patients to seek immediate medical attention if signs of methemoglobinemia occur such as bluish lips or nails.
Leukopenia
Primaquine may cause leukopenia in patients with established granulocytopenia, such as rheumatoid arthritis and lupus erythematosus. Avoid concurrent administration of bone-marrow depressants. Discontinue the use of primaquine promptly if there is a sudden decrease in leukocyte count.
Potential Prolongation of QT Interval
Due to potential for QT interval prolongation, monitor ECG when using primaquine in patients with cardiac disease, long QT syndrome, a history of ventricular arrhythmias, uncorrected hypokalemia and/or hypomagnesemia, or bradycardia (<50 bpm), and during concomitant administration with QT interval prolonging agents (see PRECAUTIONS, Drug Interactions, ADVERSE REACTIONS, and OVERDOSAGE).
CYP2D6 Potent Inhibitors, CYP2D6 Poor Metabolizers and Treatment Failure
Based on published non-clinical reports, primaquine activity probably depends on the formation of CYP2D6 metabolite(s). Therefore, CYP2D6 polymorphism or drugs that impact CYP2D6 activity may be associated with variability in clinical response to Primaquine phosphate Tablets.
Limited published clinical data reported more elevated treatment failure rates in patients with CYP2D6 poor or intermediate metabolizer status than in patients with normal/extensive metabolizer status (see CLINICAL PHARMACOLOGY).
Where possible, consider alternative medications that are not potent CYP2D6 inhibitors. If concurrent use with Primaquine phosphate Tablets is necessary, increase monitoring for possible relapse (see PRECAUTIONS, Drug Interactions).
In case of treatment failure, after checking patient's compliance to treatment, reassess use of CYP2D6 inhibitors and assess the patient's CYP2D6 status, if feasible. For poor CYP2D6 metabolizers, alternative treatment should be considered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with primaquine. No fertility studies have been conducted with primaquine. Primaquine is reported in the literature to be a weak genotoxic agent which elicits both gene mutations1, chromosomal damage and DNA strand breaks2. The publications reported positive results in the in vitro reverse gene mutation assays using bacteria (Ames test) 3,4 and in the in vivo studies using rodents (mouse bone marrow cell sister chromatid exchange, mouse bone marrow cell chromosome abnormality, and rat DNA strand breaks in multiple organs) 2, 5. The genotoxicity data obtained in vitro and in rodent models are suggestive of a human risk for genotoxicity with primaquine administration (see WARNINGS, Usage in Pregnancy).
Animal Pharmacology and/or Animal Toxicology
Literature data on reproductive toxicology identified embryo-fetal development toxicity. In studies in rats, teratogenic effects on fetus were observed (see WARNINGS, Usage in Pregnancy).
In the first reproductive toxicity study6, primaquine was administered orally to rats between gestation day (GD) 6 and GD15 at dose levels of 10.3, 30.8 and 61.5 mg/kg/day (as base) (representing approximatively 7, 20 and 40 times the human dose [HD] on a body surface area comparison) when considering a human body weight of 60 kg). High dose levels induced death of pregnant females in almost all cases, while lower dose levels caused maternal toxicity. At cesarean section, embryo resorption, a decrease in fetal survival rate and body size, internal abnormalities (including hydrocephalia, heterotaxia), and an increase in skeletal variations were observed at the mid dose-level. There were no fetal abnormalities at the low dose level providing a potential safety margin of at least 7 times the recommended clinical dose.
For the second reproductive toxicity study7, 6 to10 animals per group were used. Dose levels of 0.57, 5.7, 11.4 and 34 mg/kg/day of primaquine (as base) (representing approximatively 0.4, 4, 7 and 22 times the HD on a body surface area comparison) were administered orally to Sprague Dawley rats between GD8 and GD16, or of 57 mg/kg only once on GD13 (representing more than 37 times the HD on a body surface area comparison). A total of 1/7 and 4/6 pregnant females at 34 mg/kg/day and at 57 mg/kg, respectively, died. Primaquine-associated teratogenic malformations (including cleft palate and small chin) were observed in 4/54 fetuses in the 57 mg/kg single-dose group.
Drug Interactions
Quinacrine
Concurrent use of quinacrine (mepacrine) and Primaquine phosphate Tablets are contraindicated. Increased toxicity was seen when quinacrine was used with pamaquine, another 8-aminoquinoline (see CONTRAINDICATIONS).
Hemolytic Agents and Methemoglobinemia-Inducing Drugs
The concurrent administration of hemolytic agents or methemoglobinemia-inducing drugs and primaquine should be avoided (see PRECAUTIONS). If the concurrent administration cannot be avoided, close blood monitoring is required.
QT Interval Prolonging Drugs
The pharmacodynamic interaction potential to prolong the QT interval of the electrocardiogram between Primaquine phosphate Tablets and other drugs that effect cardiac conduction is unknown. If Primaquine phosphate Tablets are used concomitantly with other drugs that prolong the QT interval, close and frequent electrocardiogram monitoring is advised (see PRECAUTIONS, ADVERSE REACTIONS, and OVERDOSAGE).
Effects of Other Drugs on the Pharmacokinetics of Primaquine
Potent CYP2D6 Inhibitors
Published clinical and non-clinical reports indicate reduced CYP2D6 activity may decrease the formation of active metabolites of primaquine, which may reduce antimalarial efficacy of Primaquine phosphate Tablets. Where possible, consider alternative medications that are not potent CYP2D6 inhibitors. If concurrent use with Primaquine phosphate Tablets is necessary, increase monitoring for possible relapse.
Effects of Primaquine on the Pharmacokinetics of Other Drugs
CYP1A2 Substrates
Published clinical and non-clinical reports indicate primaquine inhibits CYP1A2 enzyme activity and thus may lead to increased exposure of CYP1A2 substrate drugs (e.g., duloxetine, alosetron, theophylline and tizanidine) when co-administered with Primaquine phosphate Tablets. Since data are limited, no predictions can be made regarding the extent of the impact on CYP1A2 substrate drug exposures. Increase monitoring for adverse reactions associated with the CYP1A2 substrate drug when concurrently administered with Primaquine phosphate tablets.
P-gp Substrates with Narrow Therapeutic Index
In vitro observations suggest that primaquine inhibits the P-gp membrane transporter. Therefore, there is a potential for increased concentrations of drugs that are P-gp substrates when co-administered with Primaquine phosphate Tablets. Increase monitoring for adverse reactions associated with narrow therapeutic index drugs that are P-gp substrates (e.g., digoxin and dabigatran) when concomitantly administered with Primaquine phosphate Tablets.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Primaquine phosphate Tablets are contraindicated in breastfeeding women when the infant is found to be G6PD deficient or the G6PD status of the infant is unknown (see CONTRAINDICATIONS and WARNINGS, Nursing Mothers).
Geriatric Use
Clinical studies of Primaquine phosphate Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Hepatic Impairment
Efficacy and safety of Primaquine phosphate Tablets after repeated dosing have not been assessed in patients with hepatic impairment. Primaquine is metabolized in the liver to generate active metabolites, and it is not known if efficacy could be affected in patients with hepatic impairment. Because of limited data, there is no specific dosing adjustment. If Primaquine phosphate Tablets are administered to such patients, monitoring of efficacy and for primaquine-related adverse reactions is needed, in particular in patients with severe hepatic impairment (see CLINICAL PHARMACOLOGY).
Renal Impairment
The efficacy and safety of Primaquine phosphate Tablets after repeated dosing have not been assessed in patients with renal impairment. Because of limited data, there is no specific dosing adjustment. If Primaquine phosphate Tablets are administered to such patients, monitoring of efficacy and for primaquine-related adverse reactions is needed, in particular in patients with severe renal impairment (see CLINICAL PHARMACOLOGY).
Adverse Reactions/Side Effects
Gastrointestinal: Nausea, vomiting, epigastric distress, abdominal cramps.
Hematologic: Leukopenia, hemolytic anemia, decreased hemoglobin, methemoglobinemia.
Hemolytic anemia occurs commonly in patients with G6PD deficiency and may be severe or fatal in patients with severe G6PD deficiency (see WARNINGS).
Methemoglobin levels are usually <10%, but methemoglobinemia may be severe in nicotinamide adenine dinucleotide (NADH) methemoglobin reductase deficient individuals or in patients with other risk factors (see PRECAUTIONS).
Leukopenia was observed in patients with rheumatoid arthritis or lupus erythematosus (see PRECAUTIONS).
Cardiac: Cardiac arrhythmia and QT interval prolongation (see PRECAUTIONS, OVERDOSAGE).
Nervous System: Dizziness.
Skin and Soft Tissue: Rash, pruritus.
Related/similar drugs
Overdosage
Symptoms of overdosage of primaquine phosphate include abdominal cramps, vomiting, burning epigastric distress, central nervous system disturbances including headache, insomnia, and cardiovascular disturbances, including cardiac arrhythmia and QT interval prolongation, methemoglobinemia (indicated by cyanosis), moderate leukocytosis or leukopenia, granulocytopenia, and anemia. Acute hemolysis may occur with particular severity in G6PD deficient patients.
Management
Treatment of overdosage consists of institution of appropriate symptomatic and/or supportive therapy. Consider contacting a poison center or a medical toxicologist for overdosage management recommendations.
Primaquine Dosage and Administration
Primaquine phosphate Tablets are recommended only for the radical cure of vivax malaria, the prevention of relapse in vivax malaria, or following the termination of chloroquine phosphate suppressive therapy in an area where vivax malaria is endemic. Patients suffering from an attack of vivax malaria or having parasitized red blood cells should receive a course of chloroquine phosphate, which quickly destroys the erythrocytic parasites and terminates the paroxysm. Primaquine phosphate Tablets should be administered concurrently to eradicate the exoerythrocytic parasites in adults at a dosage of 1 tablet (equivalent to 15 mg base) daily for 14 days.
Primaquine phosphate Tablets can be taken with or without food. Administration of Primaquine phosphate Tablets with food may reduce the incidence of gastrointestinal symptoms.
How is Primaquine supplied
Primaquine phosphate USP Tablets are solid oral formulation round purple tablet debossed "BY4" available in 26.3 mg and 100 count.
Available in bottles of 100 tablets. (NDC 76385-102-01)
Store at 25° C (77° F); excursions permitted to 15° - 30° C (59° - 86° F) [see USP Controlled Room Temperature].
Dispense in tight, light-resistant container as defined in the USP/NF.
Clinical Studies
Persons with acute attacks of vivax malaria, provoked by the release of erythrocytic forms of the parasite, respond readily to therapy, particularly to Chloroquine Phosphate. Primaquine eliminates tissue (exoerythrocytic) infection and prevents relapses in experimentally induced vivax malaria in human volunteers and in persons with naturally occurring infections and is a valuable adjunct to conventional therapy in vivax malaria.
To report SUSPECTED ADVERSE REACTIONS, contact Unichem Pharmaceuticals (USA) Inc. at 1-866-562-4616 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References
- Shubber EK, Jacobson-Kram D, Williams JR. Comparison of the Ames assay and the induction of sister chromatid exchanges: results with ten pharmaceuticals and five selected agents. Cell Biol Toxicol. 1986;2:379-99.
- Chatterjee T, Muhkopadhyay A, Khan KA, Giri AK. Comparative mutagenic and genotoxic effects of three antimalarial drugs, chloroquine, primaquine and amodiaquine. Mutagenesis. 1998;13:619-24.
- Marss TC. Bright JE, Morris BC. Methemoglobinogenic potential of primaquine and its mutagenicity in the Ames test. Toxicol Lett. 1987;36:281-7.
- Ono T, Norimatsu M, Yoshimura H. Mutagenic evaluation of primaquine, pentaquine and pamaquine in the Salmonella/mammalian microsome assay. Mutat Res. 1994;325:7-10.
- Giovanella F, Ferreira GK, de Prá1 SDT, et al. Effects of primaquine and chloroquine on oxidative stress parameters in rats. An Acad Bras Cienc (Annals of the Brazilian Academy of Sciences). 2015;87:1487-1496.
- Trutter JA, Reno FE, Durloo RS. Teratogenicity studies with a candidate antileishmanial drug. The Toxicologist. 1983;3:65.
- Beveridge E, Caldwell IC, Latter VS, Neal RA, Udall V, Waldron MM. The activity against Trypanosoma cruzi and cutaneous leishmaniasis, and toxicity, of moxipraquine (349C59). Trans R Soc Trop Med Hyg. 1980;74:43-51.
Manufactured For:
Manufactured for:
UNICHEM PHARMACEUTICALS (USA), INC.
East Brunswick, NJ 08816.
Revised 03/2025
| PRIMAQUINE PHOSPHATE
primaquine phosphate tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Unichem Pharmaceuticals (USA), Inc. (181620514) |
| Registrant - Unichem Laboratories Limited, India (650055882) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cerovene, Inc | 790387927 | MANUFACTURE(76385-102) | |
More about primaquine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antimalarial quinolines
- Breastfeeding
- En español


