Prenatal Vitamins Plus Low Iron: Package Insert / Prescribing Info
Package insert / product label
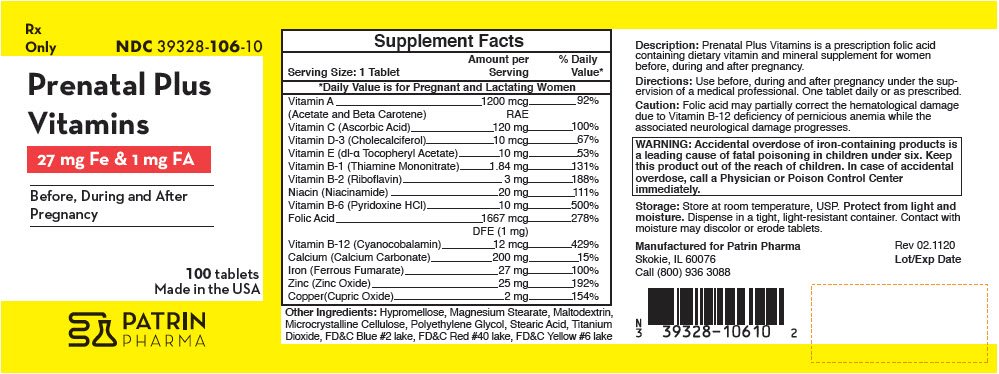
Generic name: vitamin a acetate, beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, iron, zinc and copper
Dosage form: tablet
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Jan 13, 2025.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under six. Keep this product out of the reach of children. In case of accidental overdose, call a Physician or Poison Control Center immediately.
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | Amount per Serving | % Daily Value* |
|
||
| Vitamin A | 1200 mcg | 92% |
| (Acetate and Beta Carotene) | RAE | |
| Vitamin C (Ascorbic Acid) | 120 mg | 100% |
| Vitamin D-3 (Cholecalciferol) | 10 mcg | 67% |
| Vitamin E (dl-α Tocopheryl Acetate) | 10 mg | 53% |
| Vitamin B-1 (Thiamine Mononitrate) | 1.84 mg | 131% |
| Vitamin B-2 (Riboflavin) | 3 mg | 188% |
| Niacin (Niacinamide) | 20 mg | 111% |
| Vitamin B-6 (Pyridoxine HCl) | 10 mg | 500% |
| Folic Acid | 1667 mcg | 278% |
| DFE (1 mg) | ||
| Vitamin B-12 (Cyanocobalamin) | 12 mcg | 429% |
| Calcium (Calcium Carbonate) | 200 mg | 15% |
| Iron (Ferrous Fumarate) | 27 mg | 100% |
| Zinc (Zinc Oxide) | 25 mg | 192% |
| Copper(Cupric Oxide) | 2 mg | 154% |
Other Ingredients: Hypromellose, Magnesium Stearate, Maltodextrin, Microcrystalline Cellulose, Polyethylene Glycol, Stearic Acid, Titanium Dioxide, FD&C Blue #2 lake, FD&C Red #40 lake, FD&C Yellow #6 lake
Prenatal Vitamins Plus Low Iron Description
Prenatal Plus Vitamins is a prescription folic acid containing dietary vitamin and mineral supplement for women before, during and after pregnancy.
Prenatal Vitamins Plus Low Iron Dosage and Administration
Use before, during and after pregnancy under the supervision of a medical professional. One tablet daily or as prescribed.
Precautions
Folic acid may partially correct the hematological damage due to Vitamin B-12 deficiency of pernicious anemia while the associated neurological damage progresses.
| PRENATAL PLUS VITAMINS
prenatal with ferrous fumarate and folic acid tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Patrin Pharma, Inc. (806841677) |
More about multivitamin, prenatal
- Check interactions
- Compare alternatives
- Reviews (100)
- Drug images
- Side effects
- Dosage information
- Drug class: iron products
Patient resources
Professional resources
Other brands
Prenatal 19, M-Natal Plus, PreNexa, CitraNatal Assure, ... +36 more