Prenatal Plus: Package Insert / Prescribing Info
Package insert / product label
Generic name: vitamin a acetate, beta carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide and cupric oxide
Dosage form: tablet, film coated
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Apr 7, 2025.
The Prenatal Plus brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
See also: Prenatal Plus Iron, PreNatal 19, prenatal multivitamins
On This Page
Prenatal Plus Description
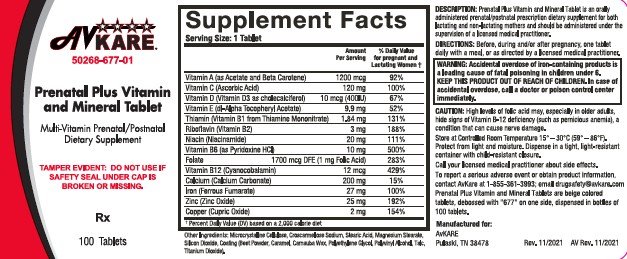
Prenatal Plus Vitamin and Mineral Tablet is an orally administered prenatal / postnatal prescription dietary supplement for both lactating and non-lactating mothers and should be administered under the supervision of a licensed medical practitioner.
Indications and Usage for Prenatal Plus
To provide Vitamin and Mineral supplementation throughout pregnancy and during the postnatal period for both the lactating and non-lactating mother. It is also useful for improving nutritional status prior to conception.
Warnings
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under age 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or Poison Control Center immediately. |
Precautions
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Call your licensed medical practitioner about side effects.
To report a serious adverse event or obtain product information, contact AvKARE at 1-855-361-3993; email drugsafety@avkare.com
Related/similar drugs
Prenatal Plus Dosage and Administration
Before, during and/or after pregnancy, one tablet daily with a meal, or as directed by a licensed medical practitioner.
| PRENATAL PLUS
vitamin a acetate, .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - AvPAK (832926666) |
More about Prenatal Plus (multivitamin, prenatal)
- Check interactions
- Compare alternatives
- Reviews (13)
- Drug images
- Side effects
- Dosage information
- Drug class: iron products
Professional resources
Other brands
Prenatal 19, M-Natal Plus, PreNexa, CitraNatal Assure, ... +34 more