Pergolide: Package Insert / Prescribing Info
Package insert / product label
Generic name: pergolide mesylate
Dosage form: tablet
Drug class: Dopaminergic antiparkinsonism agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Pergolide Description
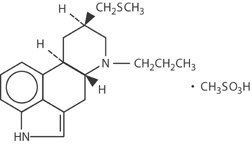
Pergolide mesylate is an ergot derivative dopamine receptor agonist at both D1 and D2 receptor sites. Pergolide mesylate is chemically designated as 8ß[(methylthio)methyl]-6-propylergoline monomethanesulfonate; the structural formula is as follows:
The empirical formula is C19H26N2S•CH4O3S, representing a molecular weight of 410.60.
Pergolide mesylate is provided for oral administration in tablets containing 0.05 mg (0.159 µmol), 0.25 mg (0.795 µmol), or 1 mg (3.18 µmol) pergolide as the base. The tablets also contain lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium starch glycolate. The 0.05 mg tablet also contains ferric oxide yellow. The 0.25 mg tablet also contains FD&C Blue No. 2 aluminum lake and ferric oxide yellow. The 1 mg tablet also contains ferric oxide red.
Pergolide - Clinical Pharmacology
Pharmacodynamic Information
Pergolide mesylate is a potent dopamine receptor agonist. Pergolide is 10 to 1000 times more potent than bromocriptine on a milligram per milligram basis in various in vitro and in vivo test systems. Pergolide mesylate inhibits the secretion of prolactin in humans; it causes a transient rise in serum concentrations of growth hormone and a decrease in serum concentrations of luteinizing hormone. In Parkinson’s disease, pergolide mesylate is believed to exert its therapeutic effect by directly stimulating post-synaptic dopamine receptors in the nigrostriatal system.
Pharmacokinetic Information (Absorption, Distribution, Metabolism and Elimination)
Information on oral systemic bioavailability of pergolide mesylate is unavailable because of the lack of a sufficiently sensitive assay to detect the drug after the administration of a single dose. However, following oral administration of 14C radiolabeled pergolide mesylate, approximately 55% of the administered radioactivity can be recovered from the urine and 5% from expired CO2, suggesting that a significant fraction is absorbed. Nothing can be concluded about the extent of presystemic clearance, if any.
Data on postabsorption distribution of pergolide are unavailable.
At least 10 metabolites have been detected, including N-despropylpergolide, pergolide sulfoxide, and pergolide sulfone. Pergolide sulfoxide and pergolide sulfone are dopamine agonists in animals. The other detected metabolites have not been identified and it is not known whether any other metabolites are active pharmacologically.
The major route of excretion is the kidney.
Pergolide is approximately 90% bound to plasma proteins. This extent of protein binding may be important to consider when pergolide mesylate is coadministered with other drugs known to affect protein binding.
Indications and Usage for Pergolide
Pergolide mesylate is indicated as adjunctive treatment to levodopa/carbidopa in the management of the signs and symptoms of Parkinson’s disease.
Evidence to support the efficacy of pergolide mesylate as an antiparkinsonian adjunct was obtained in a multicenter study enrolling 376 patients with mild to moderate Parkinson’s disease who were intolerant to l-dopa/carbidopa as manifested by moderate to severe dyskinesia and/or on-off phenomena. On average, the patients evaluated had been on l-dopa/carbidopa for 3.9 years (range, 2 days to 16.8 years). The administration of pergolide mesylate permitted a 5% to 30% reduction in the daily dose of l-dopa. On average, these patients treated with pergolide mesylate maintained an equivalent or better clinical status than they exhibited at baseline.
Contraindications
Pergolide mesylate is contraindicated in patients who are hypersensitive to this drug or other ergot derivatives.
Warnings
Falling Asleep During Activities of Daily Living
Patients treated with pergolide mesylate have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles which sometimes resulted in accidents. Although many of these patients reported somnolence while on pergolide mesylate, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events had been reported as late as 1 year after the initiation of treatment.
Somnolence is a common occurrence in patients receiving pergolide mesylate. Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with pergolide mesylate, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with pergolide mesylate such as concomitant sedating medications of the presence of sleep disorders. If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require participation (e.g., conversations, eating, etc.), pergolide mesylate should ordinarily be discontinued. If a decision is made to continue pergolide mesylate, patients should be advised to not drive and to avoid other potentially dangerous activities.
While dose reduction may reduce the degree of somnolence, there is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Symptomatic Hypotension
In clinical trials, approximately 10% of patients taking pergolide mesylate with l-dopa versus 7% taking placebo with l-dopa experienced symptomatic orthostatic and/or sustained hypotension, especially during initial treatment. With gradual dosage titration, tolerance to the hypotension usually develops. It is therefore important to warn patients of the risk, to begin therapy with low doses, and to increase the dosage in carefully adjusted increments over a period of 3 to 4 weeks (see DOSAGE AND ADMINISTRATION).
Hallucinosis
In controlled trials, pergolide mesylate with l-dopa caused hallucinosis in about 14% of patients as opposed to 3% taking placebo with l-dopa. This was of sufficient severity to cause discontinuation of treatment in about 3% of those enrolled; tolerance to this untoward effect was not observed.
Fatalities
In the placebo-controlled trial, 2 of 187 patients treated with placebo died as compared with 1 of 189 patients treated with pergolide mesylate. Of the 2,299 patients treated with pergolide mesylate in premarketing studies evaluated as of October 1988, 143 died while on the drug or shortly after discontinuing it. Because the patient population under evaluation was elderly, ill, and at high risk for death, it seems unlikely that pergolide mesylate played any role in these deaths, but the possibility that pergolide shortens survival of patients cannot be excluded with absolute certainty.
In particular, a case-by-case review of the clinical course of the patients who died failed to disclose any unique set of signs, symptoms, or laboratory results that would suggest that treatment with pergolide caused their deaths. Sixty-eight percent (68%) of the patients who died were 65 years of age or older. No death (other than a suicide) occurred within the first month of treatment; most of the patients who died had been on pergolide for years. A relative frequency of the causes of death by organ system are: Pulmonary failure/Pneumonia, 35%; Cardiovascular, 30%; Cancer, 11%; Unknown, 8.4%; Infection, 3.5%; Extrapyramidal syndrome, 3.5%; Stroke, 2.1%; Dysphagia, 2.1%; Injury, 1.4%; Suicide, 1.4%; Dehydration, 0.7%; Glomerulonephritis, 0.7%.
Serous Inflammation and Fibrosis
There have been rare reports of pleuritis, pleural effusion, pleural fibrosis, pericarditis, pericardial effusion, cardiac valvulopathy involving one or more valves, or retroperitoneal fibrosis in patients taking pergolide. In some cases, symptoms or manifestations of cardiac valvulopathy improved after discontinuation of pergolide. Pergolide should be used with caution in patients with a history of these conditions, particularly those patients who experienced the events while taking ergot derivatives. Patients with a history of such events should be carefully monitored clinically and with appropriate radiographic and laboratory studies while taking pergolide.
Precautions
Caution should be exercised when administering pergolide mesylate to patients prone to cardiac dysrhythmias.
In a study comparing pergolide mesylate and placebo, patients taking pergolide mesylate were found to have significantly more episodes of atrial premature contractions (APCs) and sinus tachycardia.
The use of pergolide mesylate in patients on l-dopa may cause and/or exacerbate preexisting states of confusion and hallucinations (see WARNINGS) and preexisting dyskinesia. Also, the abrupt discontinuation of pergolide mesylate in patients receiving it chronically as an adjunct to l-dopa may precipitate the onset of hallucinations and confusion; these may occur within a span of several days. Discontinuation of pergolide should be undertaken gradually whenever possible, even if the patient is to remain on l-dopa.
A symptom complex resembling the neuroleptic malignant syndrome (NMS) (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy, including pergolide.
Information for Patients
Because pergolide mesylate may cause somnolence and the possibility of falling asleep during activities of daily living, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that pergolide mesylate therapy does not affect them adversely. Patients should be advised that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Due to the possible additive sedative effects, caution should also be used when patients are taking other CNS depressants in combination with pergolide mesylate.
Patients and their families should be informed of the common adverse consequences of the use of pergolide mesylate (see ADVERSE REACTIONS) and the risk of hypotension (see WARNINGS).
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy.
Patients should be advised to notify their physician if they are breast feeding an infant.
Laboratory Tests
No specific laboratory tests are deemed essential for the management of patients on pergolide mesylate. Periodic routine evaluation of all patients, however, is appropriate.
Drug Interactions
Dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthines) or metoclopramide, ordinarily should not be administered concurrently with pergolide mesylate (a dopamine agonist); these agents may diminish the effectiveness of pergolide mesylate.
Because pergolide mesylate is approximately 90% bound to plasma proteins, caution should be exercised if pergolide mesylate is coadministered with other drugs known to affect protein binding.
Carcinogenesis and Mutagenesis and Impairment of Fertility
A 2 year carcinogenicity study was conducted in mice using dietary levels of pergolide mesylate equivalent to oral doses of 0.6, 3.7, and 36.4 mg/kg/day in males and 0.6, 4.4, and 40.8 mg/kg/day in females. A 2 year study in rats was conducted using dietary levels equivalent to oral doses of 0.04, 0.18, and 0.88 mg/kg/day in males and 0.05, 0.28, and 1.42 mg/kg/day in females. The highest doses tested in the mice and rats were approximately 340 and 12 times the maximum human oral dose administered in controlled clinical trials (6 mg/day equivalent to 0.12 mg/kg/day).
A low incidence of uterine neoplasms occurred in both rats and mice. Endometrial adenomas and carcinomas were observed in rats. Endometrial sarcomas were observed in mice. The occurrence of these neoplasms is probably attributable to the high estrogen/progesterone ratio that would occur in rodents as a result of the prolactin-inhibiting action of pergolide mesylate. The endocrine mechanisms believed to be involved in the rodents are not present in humans. However, even though there is no known correlation between uterine malignancies occurring in pergolide-treated rodents and human risk, there are no human data to substantiate this conclusion.
Pergolide mesylate was evaluated for mutagenic potential in a battery of tests that included an Ames bacterial mutation assay, a DNA repair assay in cultured rat hepatocytes, an in vitro mammalian cell-gene-mutation assay in cultured L5178Y cells, and a determination of chromosome alteration in bone marrow cells of Chinese hamsters. A weak mutagenic response was noted in the mammalian cell-gene-mutation assay only after metabolic activation with rat liver microsomes. No mutagenic effects were obtained in the 2 other in vitro assays and in the in vivo assay. The relevance of these findings in humans is unknown.
A fertility study in male and female mice showed that fertility was maintained at 0.6 and 1.7 mg/kg/day but decreased at 5.6 mg/kg/day. Prolactin has been reported to be involved in stimulating and maintaining progesterone levels required for implantation in mice and, therefore, the impaired fertility at the high dose may have occurred because of depressed prolactin levels.
Usage in Pregnancy – Pregnancy Category B
Reproduction studies were conducted in mice at doses of 5, 16, and 45 mg/kg/day and in rabbits at doses of 2, 6, and 16 mg/kg/day. The highest doses tested in mice and rabbits were 375 and 133 times the 6 mg/day maximum human dose administered in controlled clinical trials. In these studies, there was no evidence of harm to the fetus due to pergolide mesylate.
There are, however, no adequate and well-controlled studies in pregnant women. Among women who received pergolide mesylate for endocrine disorders in premarketing studies, there were 33 pregnancies that resulted in healthy babies and 6 pregnancies that resulted in congenital abnormalities (3 major, 3 minor); a causal relationship has not been established. Because human data are limited and because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. The pharmacologic action of pergolide mesylate suggests that it may interfere with lactation. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions to pergolide mesylate in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Of the total number of subjects in clinical studies of pergolide mesylate, 78 were 65 and over. There were no apparent differences in efficacy between these subjects and younger subjects. There was an increased incidence of confusion, somnolence, and peripheral edema in patients 65 and over. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Adverse Reactions/Side Effects
In premarketing clinical trials, the most commonly observed adverse events associated with use of pergolide mesylate which were not seen at an equivalent incidence among placebo-treated patients were: nervous system complaints, including dyskinesia, hallucinations, somnolence, insomnia; digestive complaints, including nausea, constipation, diarrhea, dyspepsia; and respiratory system complaints, including rhinitis.
Associated with Discontinuation of Treatment
Twenty-seven percent (27%) of approximately 1,200 patients receiving pergolide mesylate for treatment of Parkinson’s disease in premarketing clinical trials in the U.S. and Canada discontinued treatment due to adverse events. The events most commonly causing discontinuation were related to the nervous system (15.5%), primarily hallucinations (7.8%) and confusion (1.8%).
Incidence in Controlled Clinical Trials
The table that follows enumerates adverse events that occurred at a frequency of 1% or more among patients taking pergolide mesylate who participated in the premarketing controlled clinical trials comparing pergolide mesylate with placebo. In a double-blind, controlled study of 6 months’ duration, patients with Parkinson’s disease were continued on l-dopa/carbidopa and were randomly assigned to receive either pergolide mesylate or placebo as additional therapy.
The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side-effect incidence rate in the population studied.
Events Observed During the Premarketing Evaluation of Pergolide Mesylate
This section reports event frequencies evaluated as of October 1988 for adverse events occurring in a group of approximately 1,800 patients who took multiple doses of pergolide mesylate. The conditions and duration of exposure to pergolide mesylate varied greatly, involving well-controlled studies as well as experience in open and uncontrolled clinical settings. In the absence of appropriate controls in some of the studies, a causal relationship between these events and treatment with pergolide mesylate cannot be determined.
The following enumeration by organ system describes events in terms of their relative frequency of reporting in the data base. Events of major clinical importance are also described in the WARNINGS and PRECAUTIONS sections.
The following definitions of frequency are used: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Body as a Whole– Frequent: headache, asthenia, accidental injury, pain, abdominal pain, chest pain, back pain, flu syndrome, neck pain, fever; Infrequent: facial edema, chills, enlarged abdomen, malaise, neoplasm, hernia, pelvic pain, sepsis, cellulitis, moniliasis, abscess, jaw pain, hypothermia; Rare: acute abdominal syndrome, LE syndrome.
Cardiovascular System– Frequent: postural hypotension, syncope, hypertension, palpitations, vasodilatations, congestive heart failure; Infrequent: myocardial infarction, tachycardia, heart arrest, abnormal electrocardiogram, angina pectoris, thrombophlebitis, bradycardia, ventricular extrasystoles, cerebrovascular accident, ventricular tachycardia, cerebral ischemia, atrial fibrillation, varicose vein, pulmonary embolus, AV block, shock; Rare: vasculitis, pulmonary hypertension, pericarditis, migraine, heart block, cerebral hemorrhage.
Digestive System– Frequent: nausea, vomiting, dyspepsia, diarrhea, constipation, dry mouth, dysphagia; Infrequent: flatulence, abnormal liver function tests, increased appetite, salivary gland enlargement, thirst, gastroenteritis, gastritis, periodontal abscess, intestinal obstruction, nausea and vomiting, gingivitis, esophagitis, cholelithiasis, tooth caries, hepatitis, stomach ulcer, melena, hepatomegaly, hematemesis, eructation; Rare: sialadenitis, peptic ulcer, pancreatitis, jaundice, glossitis, fecal incontinence, duodenitis, colitis, cholecystitis, aphthous stomatitis, esophageal ulcer.
Endocrine System– Infrequent: hypothyroidism, adenoma, diabetes mellitus, ADH inappropriate; Rare: endocrine disorder, thyroid adenoma.
Hemic and Lymphatic System– Frequent: anemia; Infrequent: leukopenia, lymphadenopathy, leukocytosis, thrombocytopenia, petechia, megaloblastic anemia, cyanosis; Rare: purpura, lymphocytosis, eosinophilia, thrombocythemia, acute lymphoblastic leukemia, polycythemia, splenomegaly.
Metabolic and Nutritional System– Frequent: peripheral edema, weight loss, weight gain; Infrequent: dehydration, hypokalemia, hypoglycemia, iron deficiency anemia, hyperglycemia, gout, hypercholesteremia; Rare: electrolyte imbalance, cachexia, acidosis, hyperuricemia.
Musculoskeletal System– Frequent: twitching, myalgia, arthralgia; Infrequent: bone pain, tenosynovitis, myositis, bone sarcoma, arthritis; Rare: osteoporosis, muscle atrophy, osteomyelitis.
Nervous System– Frequent: dyskinesia, dizziness, hallucinations, confusion, somnolence, insomnia, dystonia, paresthesia, depression, anxiety, tremor, akinesia, extrapyramidal syndrome, abnormal gait, abnormal dreams, incoordination, psychosis, personality disorder, nervousness, choreoathetosis, amnesia, paranoid reaction, abnormal thinking; Infrequent: akathisia, neuropathy, neuralgia, hypertonia, delusions, convulsion, libido increased, euphoria, emotional lability, libido decreased, vertigo, myoclonus, coma, apathy, paralysis, neurosis, hyperkinesia, ataxia, acute brain syndrome, torticollis, meningitis, manic reaction, hypokinesia, hostility, agitation, hypotonia; Rare: stupor, neuritis, intracranial hypertension, hemiplegia, facial paralysis, brain edema, myelitis, hallucinations and confusion after abrupt discontinuation.
Respiratory System– Frequent: rhinitis, dyspnea, pneumonia, pharyngitis, cough increased; Infrequent: epistaxis, hiccup, sinusitis, bronchitis, voice alteration, hemoptysis, asthma, lung edema, pleural effusion, laryngitis, emphysema, apnea, hyperventilation; Rare: pneumothorax, lung fibrosis, larynx edema, hypoxia, hypoventilation, hemothorax, carcinoma of lung.
Skin and Appendages System– Frequent: sweating, rash; Infrequent: skin discoloration, pruritus, acne, skin ulcer, alopecia, dry skin, skin carcinoma, seborrhea, hirsutism, herpes simplex, eczema, fungal dermatitis, herpes zoster; Rare: vesiculobullous rash, subcutaneous nodule, skin nodule, skin benign neoplasm, lichenoid dermatitis.
Special Senses System– Frequent: abnormal vision, diplopia; Infrequent: otitis media, conjunctivitis, tinnitus, deafness, taste perversion, ear pain, eye pain, glaucoma, eye hemorrhage, photophobia, visual field defect; Rare: blindness, cataract, retinal detachment, retinal vascular disorder.
Urogenital System– Frequent: urinary tract infection, urinary frequency, urinary incontinence, hematuria, dysmenorrhea; Infrequent: dysuria, breast pain, menorrhagia, impotence, cystitis, urinary retention, abortion, vaginal hemorrhage, vaginitis, priapism, kidney calculus, fibrocystic breast, lactation, uterine hemorrhage, urolithiasis, salpingitis, pyuria, metrorrhagia, menopause, kidney failure, breast carcinoma, cervical carcinoma; Rare: amenorrhea, bladder carcinoma, breast engorgement, epididymitis, hypogonadism, leukorrhea, nephrosis, pyelonephritis, urethral pain, uricaciduria, withdrawal bleeding.
Postintroduction Reports - Voluntary reports of adverse events temporally associated with pergolide that have been received since market introduction and which may have no causal relationship with the drug, include the following: neuroleptic malignant syndrome.
Related/similar drugs
Overdosage
There is no clinical experience with massive overdosage. The largest overdose involved a young hospitalized adult patient who was not being treated with pergolide mesylate but who intentionally took 60 mg of the drug. He experienced vomiting, hypotension, and agitation. Another patient receiving a daily dosage of 7 mg of pergolide mesylate unintentionally took 19 mg/day for 3 days, after which his vital signs were normal but he experienced severe hallucinations. Within 36 hours of resumption of the prescribed dosage level, the hallucinations stopped. One patient unintentionally took 14 mg/day for 23 days instead of her prescribed 1.4 mg/day dosage. She experienced severe involuntary movements and tingling in her arms and legs. Another patient who inadvertently received 7 mg instead of the prescribed 0.7 mg experienced palpitations, hypotension, and ventricular extrasystoles. The highest total daily dose (prescribed for several patients with refractory Parkinson’s disease) has exceeded 30 mg.
Symptons
Animal studies indicate that the manifestations of overdosage in man might include nausea, vomiting, convulsions, decreased blood pressure, and CNS stimulation. The oral median lethal doses in mice and rats were 54 and 15 mg/kg respectively.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
Management of overdosage may require supportive measures to maintain arterial blood pressure. Cardiac function should be monitored; an antiarrhythmic agent may be necessary. If signs of CNS stimulation are present, a phenothiazine or other butyrophenone neuroleptic agent may be indicated; the efficacy of such drugs in reversing the effects of overdose has not been assessed.
Protect the patient’s airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient’s vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient’s airway when employing gastric emptying or charcoal.
There is no experience with dialysis or hemoperfusion, and these procedures are unlikely to be of benefit.
Pergolide Dosage and Administration
Administration of pergolide mesylate tablets should be initiated with a daily dosage of 0.05 mg for the first 2 days. The dosage should then be gradually increased by 0.1 or 0.15 mg/day every third day over the next 12 days of therapy. The dosage may then be increased by 0.25 mg/day every third day until an optimal therapeutic dosage is achieved.
Pergolide mesylate tablets are usually administered in divided doses 3 times per day. During dosage titration, the dosage of concurrent l-dopa/carbidopa may be cautiously decreased.
In clinical studies, the mean therapeutic daily dosage of pergolide mesylate tablets was 3 mg/day. The average concurrent daily dosage of l-dopa/ carbidopa (expressed as l-dopa) was approximately 650 mg/day. The efficacy of pergolide mesylate tablets at doses above 5 mg/day has not been systematically evaluated.
How is Pergolide supplied
Pergolide mesylate tablets, equivalent to 0.05 mg pergolide, are available as ivory, capsule-shaped tablets, scored on one side and debossed with “9” on the left side of the score and “3” on the right side of the score. The other side is debossed with “7160”. They are available in bottles of 100 (NDC 49884-316-01).
Pergolide mesylate tablets, equivalent to 0.25 mg pergolide, are available as mottled green, capsule-shaped tablets, scored on one side and debossed with “9” on the left side of the score and “3” on the right side of the score. The other side is debossed with “7159”. They are available in bottles of 100 (NDC 49884-317-01).
Pergolide mesylate tablets, equivalent to 1 mg pergolide, are available as mottled pink, capsule-shaped tablets, scored on one side and debossed with “9” on the left side of the score and “3” on the right side of the score. The other side is debossed with “7161”. They are available in bottles of 100 (NDC 49884-318-01).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
| PERGOLIDE MESYLATE
pergolide mesylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PERGOLIDE MESYLATE
pergolide mesylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PERGOLIDE MESYLATE
pergolide mesylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Par Pharmaceutical Inc. (092733690) |
| Registrant - Par Pharmaceutical, Inc. (092733690) |
More about pergolide
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: dopaminergic antiparkinsonism agents