Brekiya: Package Insert / Prescribing Info
Package insert / product label
Generic name: dihydroergotamine mesylate
Dosage form: injection
Drug class: Antimigraine agents
Medically reviewed by Drugs.com. Last updated on May 27, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Drug Abuse and Dependence
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- How Supplied/Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
These highlights do not include all the information needed to use BREKIYA® safely and effectively. See full prescribing information for BREKIYA®.
BREKIYA® (dihydroergotamine mesylate) injection, for subcutaneous use
Initial U.S. Approval: 1946
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
See full prescribing information for complete boxed warning.
Serious and/or life-threatening peripheral ischemia has been associated with the co-administration of dihydroergotamine with strong CYP3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of BREKIYA with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)].
Indications and Usage for Brekiya
BREKIYA is an ergotamine derivative indicated for the acute treatment of migraine with or without aura and the acute treatment of cluster headaches in adults. (1)
Limitations of Use
BREKIYA is not indicated for the preventive treatment of migraine or for the management of hemiplegic migraine or migraine with brainstem aura. (1)
Brekiya Dosage and Administration
- For subcutaneous injection only. (2.1)
- Recommended dosage is 1 mg administered subcutaneously as a single 1 mL autoinjector. (2.1)
- Do not exceed 3 mg (3 doses) in a 24-hour period. (2.1)
- Do not exceed 6 mg (6 doses) in a week. (2.1)
- Prior to initiation, a cardiovascular evaluation is recommended. (2.2)
Dosage Forms and Strengths
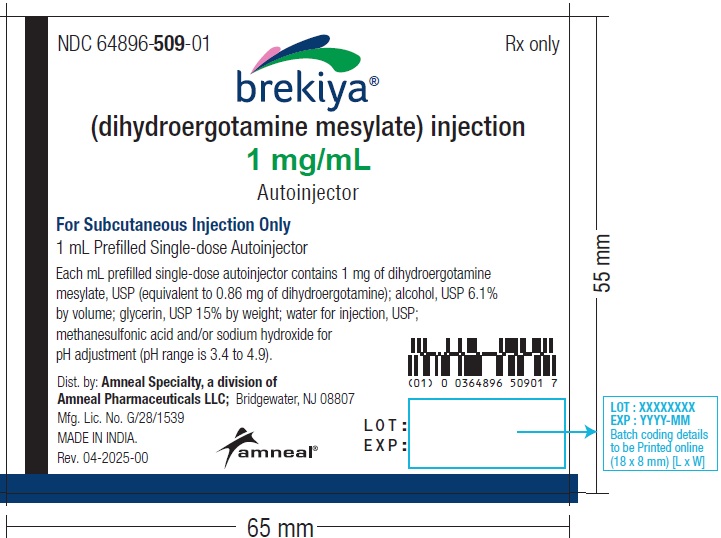
Injection: 1 mg/mL dihydroergotamine mesylate as a 1 mL single-dose autoinjector. (3)
Contraindications
- Concomitant use of strong CYP3A4 inhibitors (4)
- Ischemic heart disease or coronary artery vasospasm (4)
- Uncontrolled hypertension, peripheral arterial diseases, sepsis, following vascular surgery, or severe hepatic or renal impairment (4)
- Concomitant use of other 5-HT1 agonists or ergotamine-containing or ergot-type medications within 24 hours (4)
- Hypersensitivity to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in BREKIYA (4)
- Concomitant use with peripheral and central vasoconstrictors (4)
Warnings and Precautions
- Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities: In patients with risk factors predictive of coronary artery disease, consider first dose administration under medical supervision with an electrocardiogram. (5.2)
- Cerebrovascular Adverse Reactions and Fatalities: Cerebrovascular hemorrhage, subarachnoid hemorrhage, and stroke have been reported; discontinue BREKIYA if suspected. (5.3)
- Other Vasospasm Related Adverse Reaction: BREKIYA may cause vasospasm or elevation in blood pressure. Discontinue if signs or symptoms of vasoconstriction develop. (5.4, 5.5)
- Medication Overuse Headache: Detoxification may be necessary. (5.6)
- Preterm Labor: Advise pregnant women of the risk. (5.7, 8.1)
- Fibrotic Complication: Pleural and retroperitoneal fibrosis have been reported following prolonged daily use of dihydroergotamine mesylate. Do not exceed the BREKIYA dosing guidelines or use for chronic daily administration. (5.8)
Adverse Reactions/Side Effects
Serious cardiac events (including fatal) that have been reported with dihydroergotamine mesylate injection use include coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, ventricular fibrillation. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals LLC at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
Full Prescribing Information
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of BREKIYA with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7.1)].
1. Indications and Usage for Brekiya
BREKIYA is indicated for the acute treatment of migraine with or without aura and the acute treatment of cluster headaches in adults.
Limitations of Use
BREKIYA is not indicated for the preventive treatment of migraine.
BREKIYA is not indicated for the management of hemiplegic migraine or migraine with brainstem aura.
2. Brekiya Dosage and Administration
2.1 Recommended Dosage
BREKIYA autoinjector is for subcutaneous injection only.
The recommended dose of BREKIYA is 1 mg administered subcutaneously via a single 1 mL autoinjector. The dose may be repeated, as needed, at 1-hour intervals to a total maximum of 3 mg (3 doses) in a 24-hour period.
Do not exceed 6 mg (6 doses) total in a week.
2.2 Assessment Prior to First Dose
Prior to initiation of BREKIYA, a cardiovascular evaluation is recommended [see Warnings and Precautions (5.3)]. For patients with risk factors predictive of coronary artery disease who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of BREKIYA take place in the setting of an equipped healthcare facility.
2.3 Important Administration Instructions
See the “Instructions for Use” for detailed steps for administering the subcutaneous injection using the autoinjector.
Preparation
- BREKIYA is a prefilled, single-dose disposable autoinjector intended to be given subcutaneously into the skin of the middle thigh.
- Rotate injection sites. Choose an injection site at least 2 inches away from the last injection site.
- Do not inject into moles, scars, birthmarks, or areas where the skin is tender, bruised, red, or hard.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Carefully examine the liquid medication for discoloration, cloudiness, floating flakes or particles. Administer only if clear and colorless.
Administration
- Remove the red needle cap by pulling it straight off.
- Position the autoinjector straight onto the cleaned injection site with the white safety guard resting on the skin. Push the autoinjector down and press and release the gray activation button to start the injection. A “click” will sound when the injection starts.
- The autoinjector takes approximately 10 seconds to deliver the dose; when the viewing window is fully blocked (completely blue), the full dose has been administered.
3. Dosage Forms and Strengths
Injection: 1 mg/mL dihydroergotamine mesylate as a clear, colorless solution in a 1 mL single-dose autoinjector.
4. Contraindications
BREKIYA is contraindicated in patients:
- with concomitant use of strong CYP3A4 inhibitors [see Warnings and Precautions (5.1) and Drug Interactions (7.1)]
- with ischemic heart disease (e.g., angina pectoris, history of myocardial infarction, or documented silent ischemia) or patients who have clinical symptoms or findings consistent with coronary artery vasospasm, including Prinzmetal's variant angina [see Warnings and Precautions (5.4)]
- with uncontrolled hypertension [see Warnings and Precautions (5.5)]
- with peripheral arterial disease
- with sepsis
- following vascular surgery
- with severe hepatic impairment
- with severe renal impairment
- with known hypersensitivity to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in BREKIYA [see Warnings and Precautions (5.9)]
- with recent use (i.e., within 24 hours) of other 5-HT1 agonists, ergotamine-containing or ergot-type medications [see Drug Interactions (7.2)]
- with concomitant use of peripheral and central vasoconstrictors because the combination may result in additive or synergistic elevation of blood pressure [see Warnings and Precautions (5.5)]
5. Warnings and Precautions
5.1 Peripheral Ischemia Following Coadministration with Strong CYP3A4 Inhibitors
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine and strong CYP3A4 inhibitors. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or and ischemia of the extremities is increased. Hence, concomitant use of BREKIYA with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4) and Drug Interactions (7.1)].
5.2 Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities
The potential for cardiac adverse reactions exists with BREKIYA treatment. Serious adverse cardiac events, including some that have been fatal, have occurred following use of dihydroergotamine mesylate. These events have included acute myocardial infarction, life-threatening disturbances of cardiac rhythm (e.g., ventricular tachycardia and ventricular fibrillation), coronary artery vasospasm, and transient myocardial ischemia.
Prior to initiation of BREKIYA, a cardiovascular evaluation is recommended to determine if the patient is free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. If, during the cardiovascular evaluation, the patient’s medical history (including risk factors), or electrocardiographic investigation findings are consistent with coronary artery vasospasm or myocardial ischemia, BREKIYA should not be administered [see Contraindications (4)].
For patients with risk factors predictive of coronary artery disease (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of coronary artery disease, females who are surgically or physiologically postmenopausal, or males who are over 40 years of age) who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of BREKIYA take place in the setting of an equipped healthcare facility, unless the patient has previously received dihydroergotamine. During the interval immediately following the first use of BREKIYA, an electrocardiogram is recommended in those patients with risk factors because ischemia can occur in the absence of clinical symptoms.
5.3 Cerebrovascular Adverse Reactions and Fatalities
The potential for adverse cerebrovascular events exists with BREKIYA treatment. Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with dihydroergotamine mesylate; and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the dihydroergotamine mesylate having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). Discontinue BREKIYA if a cerebrovascular event is suspected.
5.4 Other Vasospasm-Related Adverse Reactions
BREKIYA, like other ergot alkaloids, may cause vasospastic reactions other than coronary artery vasospasm. Myocardial, peripheral vascular, and colonic ischemia have been reported with dihydroergotamine mesylate.
Dihydroergotamine associated vasospastic phenomena may also cause muscle pains, numbness, coldness, pallor, and cyanosis of the digits. In patients with compromised circulation, persistent vasospasm may result in gangrene or death. BREKIYA should be discontinued immediately if signs or symptoms of vasoconstriction develop.
Patients who experience other symptoms or signs suggestive of decreased arterial flow, such as ischemic bowel syndrome or Raynaud’s syndrome following the use of any 5-HT agonist, including BREKIYA, should be evaluated by a healthcare provider.
5.5 Increase in Blood Pressure
Significant elevation in blood pressure has been reported on rare occasions in patients with and without a history of hypertension treated with dihydroergotamine mesylate. BREKIYA is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)].
An 18% increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
5.6 Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamines, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (i.e., medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients including withdrawal of the overused drugs and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Preterm Labor
Based on the mechanism of action of dihydroergotamine and findings from the published literature, BREKIYA may cause preterm labor. Avoid use of BREKIYA during pregnancy [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.2)].
5.8 Fibrotic Complications
The potential for fibrotic complications exists with BREKIYA treatment. There have been reports of pleural and retroperitoneal fibrosis in patients following prolonged daily use of dihydroergotamine mesylate. Rarely, prolonged daily use of other ergot alkaloid drugs has been associated with cardiac valvular fibrosis. Rare cases have also been reported in association with the use of dihydroergotamine mesylate; however, in those cases, patients also received drugs known to be associated with cardiac valvular fibrosis.
Administration of BREKIYA should not exceed the dosing guidelines and should not be used for chronic daily administration [see Dosage and Administration (2.1)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Peripheral Ischemia Following Coadministration with Strong CYP3A4 Inhibitors [see Boxed Warning and Warnings and Precautions (5.1)]
- Myocardial Ischemia and/or Infarction, Other Adverse Cardiac Events, and Fatalities [see Warnings and Precautions (5.2)]
- Cerebrovascular Adverse Reactions and Fatalities [see Warnings and Precautions (5.3)]
- Other Vasospasm Related Adverse Reactions [see Warnings and Precautions (5.4)]
- Increase in Blood Pressure [see Warnings and Precautions (5.5)]
- Medication Overuse Headache [see Warnings and Precautions (5.6)]
- Preterm Labor [see Warnings and Precautions (5.7)]
- Fibrotic Complications [see Warnings and Precautions (5.8)]
- Hypersensitivity [see Warnings and Precautions (5.9)]
The following adverse reactions associated with the use of dihydroergotamine were identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
Serious cardiac events, including some that have been fatal, have occurred following use of dihydroergotamine. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation [see Contraindications (4) and Warnings and Precautions (5.3)].
Fibrotic complications have been reported in association with long term use of injectable dihydroergotamine mesylate [see Warnings and Precautions (5.8)].
Vasospasm, paresthesia, hypertension, dizziness, anxiety, dyspnea, headache, flushing, diarrhea, rash, increased sweating, and pleural and retroperitoneal fibrosis occurred after long-term use of dihydroergotamine.
Cases of myocardial infarction and stroke have been reported following the use of dihydroergotamine [see Warnings and Precautions (5.2)].
Related/similar drugs
7. Drug Interactions
7.1 CYP3A4 Inhibitors
There have been rare reports of serious adverse events in connection with the coadministration of intravenous administration of dihydroergotamine and strong CYP3A4 inhibitors resulting in vasospasm that led to cerebral ischemia and/or ischemia of the extremities [see Warnings and Precautions (5.1)]. The use of strong CYP3A4 inhibitors with BREKIYA is contraindicated [see Contraindications (4)]. Administer moderate CYP3A4 inhibitors with caution.
7.2 Triptans
Triptans (serotonin [5-HT 1B/1D receptor agonists) have been reported to cause coronary artery vasospasm, and its effect could be additive with BREKIYA. Therefore, triptans and BREKIYA should not be taken within 24 hours of each other [see Contraindications (4)].
7.3 Beta Blockers
There have been reports that propranolol may potentiate the vasoconstrictive action of ergotamine by blocking the vasodilating property of epinephrine.
7.4 Vasoconstrictors
BREKIYA is contraindicated for use with peripheral and central vasoconstrictors because the combination may cause synergistic elevation of blood pressure [see Warnings and Precautions (5.5)].
7.5 Nicotine
Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy [see Warnings and Precautions (5.2, 5.4)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from published literature indicate an increased risk of preterm delivery with dihydroergotamine, the active moiety in BREKIYA, use during pregnancy. Avoid use of BREKIYA during pregnancy [see Warnings and Precautions (5.9)]. Data collected over decades have shown no increased risk of major birth defects or miscarriage with the use of dihydroergotamine mesylate during pregnancy.
In animal reproduction studies, adverse effects on development were observed following administration of dihydroergotamine mesylate during pregnancy (decreased fetal body weight and/or skeletal ossification) in rats and rabbits or during pregnancy and lactation in rats (decreased body weight and impaired reproductive function in the offspring) at doses that were not associated with maternal toxicity (see Data).
The estimated rate of major birth defects (2.2% to 2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
Data
Animal Data
Intranasal administration of dihydroergotamine mesylate to pregnant rats throughout the period of organogenesis resulted in decreased fetal body weight and/or skeletal ossification at doses of 0.16 mg/day or greater. A no-effect level for adverse effects on embryofetal toxicity was not identified in rats. Intranasal administration of dihydroergotamine mesylate to pregnant rabbits throughout organogenesis resulted in decreased skeletal ossification at 3.6 mg/day. The no-effect dose for adverse effects on embryofetal toxicity in rabbits was 1.2 mg/day.
Intranasal administration of dihydroergotamine mesylate to female rats throughout pregnancy and lactation resulted in decreased body weight and impaired reproductive function (decreased mating indices) in the offspring at doses of 0.16 mg/day or greater. A no-effect dose for adverse developmental effects in rats was not established. Effects on offspring development occurred at doses below those that produced evidence of maternal toxicity in these studies.
Dihydroergotamine-induced intrauterine growth retardation has been attributed to reduced uteroplacental blood flow resulting from prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone.
8.2 Lactation
Risk Summary
There are no data on the presence of dihydroergotamine in human milk; however, ergotamine, a related drug, is excreted in human milk. There are reports of vomiting, diarrhea, weak pulse, and unstable blood pressure in breastfed infants exposed to ergotamine. BREKIYA may reduce milk supply because it may decrease prolactin levels.
Because of the potential for reduced milk supply and serious adverse events in the breastfed infant, including diarrhea, vomiting, weak pulse, and unstable blood pressure; advise patients not to breastfeed during treatment with BREKIYA and for 3 days after the last dose. Breast milk supply during this time should be pumped and discarded.
8.5 Geriatric Use
Clinical studies of dihydroergotamine products did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
9. Drug Abuse and Dependence
9.1 Controlled Substance
BREKIYA contains dihydroergotamine (as the mesylate salt), which is not a controlled substance.
9.2 Abuse
Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Currently available data have not demonstrated drug abuse with dihydroergotamine. However, cases of drug abuse in patients on other forms of ergot therapy have been reported.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Currently available data have not demonstrated physical or psychological dependence with dihydroergotamine. However, cases of psychological dependence in patients on other forms of ergot therapy have been reported.
10. Overdosage
Symptoms
Excessive doses of dihydroergotamine may result in peripheral signs and symptoms of ergotism.
In general, the symptoms of an acute BREKIYA overdose may be similar to those of an ergotamine overdose, although there may be less pronounced nausea and vomiting with BREKIYA. The symptoms of an ergotamine overdose include the following: numbness, tingling, pain, and cyanosis of the extremities associated with diminished or absent peripheral pulses; respiratory depression; an increase and/or decrease in blood pressure, usually in that order; confusion, delirium, convulsions, and coma; and/or some degree of nausea, vomiting, and abdominal pain.
In laboratory animals, dihydroergotamine was lethal when given at intravenous doses of 44 mg/kg in mice, 130 mg/kg in rats, and 37 mg/kg in rabbits.
Treatment
Treatment includes discontinuance of the drug, local application of warmth to the affected area, the administration of vasodilators, and nursing care to prevent tissue damage. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
11. Brekiya Description
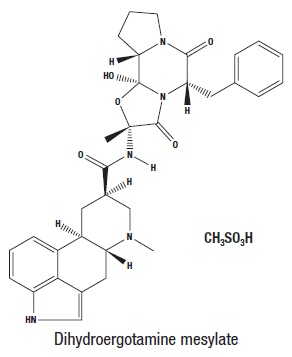
BREKIYA (dihydroergotamine mesylate) injection is an ergotamine derivative. Dihydroergotamine mesylate is a white or almost white, crystalline powder. It is slightly soluble in water and chloroform and sparingly soluble in methanol. The chemical name for dihydroergotamine mesylate is ergotaman-3´,6´,18-trione,9,10-dihydro-12´-hydroxy-2´-methyl-5´-(phenylmethyl) monomethanesulfonate (salt). Its molecular weight is 679.8g/mol and its molecular formula is C33H37N5O5∙CH4O3S.
The chemical structure is
BREKIYA is a clear, colorless, sterile solution supplied in 1 mL single-dose autoinjector for subcutaneous administration. Each mL contains 1 mg dihydroergotamine mesylate, USP (equivalent to 0.86 mg of dihydroergotamine). BREKIYA also contains ethanol, USP 6.1% by volume; glycerin, USP 15% by weight; water for injection, USP; may contain methanesulfonic acid and/or sodium hydroxide, NF for pH adjustment. pH range is 3.4 to 4.9.
12. Brekiya - Clinical Pharmacology
12.1 Mechanism of Action
Dihydroergotamine binds with high affinity to 5-HT1Dα and 5-HT1Dβ receptors. The therapeutic activity of dihydroergotamine in migraine is generally attributed to the agonist effects at 5-HT1D receptors.
12.2 Pharmacodynamics
Significant elevation in blood pressure has been reported in patients treated with dihydroergotamine with and without a history of hypertension [see Warnings and Precautions (5.5)].
Dihydroergotamine possesses oxytocic properties [see Warnings and Precautions (5.7)].
12.3 Pharmacokinetics
Absorption
Following BREKIYA administration, the mean maximum plasma concentration was 3.8 ng/mL, and the median time from dosing to maximum plasma concentration was approximately 0.4 hours.
Distribution
Dihydroergotamine mesylate is 93% plasma protein bound. The apparent steady-state volume of distribution is approximately 800 liters.
Elimination
Metabolism
Four dihydroergotamine mesylate metabolites have been identified in human plasma following oral administration. The major metabolite, 8´-β-hydroxydihydroergotamine, exhibits affinity equivalent to its parent for adrenergic and 5-HT receptors and demonstrates equivalent potency in several venoconstrictor activity models, in vivo and in vitro. The other metabolites (i.e., dihydrolysergic acid, dihydrolysergic amide, and a metabolite formed by oxidative opening of the proline ring) are of minor importance. Following nasal administration, total metabolites represent only 20% to 30% of plasma AUC. Quantitative pharmacokinetic characterization of the four metabolites has not been performed.
Excretion
The major excretory route of dihydroergotamine is via the bile in the feces. The total body clearance is 1.5 L/min which reflects mainly hepatic clearance. Only 6% to 7% of unchanged dihydroergotamine is excreted in the urine after intramuscular injection. The renal clearance (0.1 L/min) is unaffected by the route of dihydroergotamine administration. The decline of plasma dihydroergotamine after intramuscular or intravenous administration is multi-exponential with a terminal half-life of about 9 hours.
Specific Populations
No studies have been conducted on the effect of renal or hepatic impairment, gender, race, or ethnicity on dihydroergotamine pharmacokinetics [see Contraindications (4)].
Drug Interaction Studies
Pharmacokinetic interactions have been reported in patients treated orally with other ergot alkaloids (e.g., increased levels of ergotamine) and macrolide antibiotics, presumably due to inhibition of cytochrome P450 3A metabolism of the alkaloids. Dihydroergotamine has also been shown to be an inhibitor of cytochrome P450 3A catalyzed reactions and rare reports of ergotism have been obtained from patients treated with dihydroergotamine and macrolide antibiotics (e.g., clarithromycin, erythromycin), and in patients treated with dihydroergotamine and protease inhibitors (e.g., ritonavir), presumably due to inhibition of cytochrome P450 3A metabolism of ergotamine [see Contraindications (4)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year mouse carcinogenicity study, subcutaneous (SC) administration of dihydroergotamine mesylate (0, 0.5, 1.5 or 5 mg/kg/day) resulted in an increased incidence of fibrosarcoma at the injection sites in males and females at the high dose. The higher dose not associated with an increase in tumors (1.5 mg/kg/day) is approximately 2 times the recommended human dose (RHD) of 3 mg/day SC on a body surface area (mg/m2) basis.
In a 2-year rat carcinogenicity study, intranasal administration of dihydroergotamine mesylate (0, 0.4, 0.8 or 1.6 mg/day for 13 weeks, followed by 0, 0.08, 0.24 or 0.8 mg/day for the remainder of the study) did not result in an increase in tumors.
Mutagenesis
Dihydroergotamine mesylate was negative in an in vitro mutagenicity (Ames) assay and positive in in vitro chromosomal aberration (V79 Chinese hamster cell assay with metabolic activation, and human peripheral blood lymphocyte) assays. Dihydroergotamine was negative in in vivo micronucleus assays in mouse and hamster.
Impairment of Fertility
Intranasal administration of dihydroergotamine to rats at doses up to 1.6 mg/day was not associated with adverse effects on fertility.
16. How is Brekiya supplied
16.1 How Supplied
BREKIYA (dihydroergotamine mesylate) injection is a clear, colorless, sterile solution of 1 mg/mL dihydroergotamine mesylate available as:
| Dosage Unit | Package Size | NDC # |
| 1 mL prefilled single-dose autoinjector | Carton of 4 | NDC 64896-509-02 |
Caution: The rigid needle shield of the BREKIYA autoinjector contains a needle cover (located inside the cap) that contains dry natural rubber, which is made from latex [see Contraindications (4) and Warnings and Precautions (5.9)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Serious and/or Life-Threatening Reactions with Coadministration of CYP3A4 Inhibitors
Inform patients that serious and/or life-threatening peripheral ischemia (cerebral ischemia and/or ischemia of the extremities) has been associated with the coadministration of dihydroergotamine and strong CYP3A4 inhibitors, [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7.1)].
Myocardial Ischemia and/or Infarction, Other Cardiac Events, Cerebrovascular Events, and Fatalities
Inform patients of the risk for serious cardiac, cerebrovascular, and other vasospasm related events. Advise patients to notify their healthcare provider if they develop any risk factors or symptoms while taking BREKIYA. Inform patients that nicotine may provoke vasoconstriction predisposing to a greater ischemic response [see Warnings and Precautions (5.2, 5.3, 5.4)].
Increase in Blood Pressure
Inform patients of the risk for significant elevation in blood pressure [see Warnings and Precautions (5.5)].
Medication Overuse Headache
Inform patients that use of drugs to treat migraine attacks for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Hypersensitivity
Inform patients that the rigid needle shield of the BREKIYA autoinjector contains a needle cover (located inside the cap) that contains dry natural rubber, which is made from latex, and can cause allergic reactions in latex-sensitive individuals [see Warnings and Precautions (5.9)].
Drug Interactions
Advise patients to inform their healthcare providers if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions [see Drug Interactions (7)].
Pregnancy
Advise patients of the risk for preterm birth. Advise women to inform their healthcare provider if they are pregnant or intend to become pregnant [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Lactation
Advise patients not to breastfeed during treatment with BREKIYA [see Use in Specific Populations (8.2)].
Important Administration Instructions
Advise patients on the proper use of BREKIYA prior to the initial use and instruct them to read the Instructions for Use [see Dosage and Administration (2.3)].
For more information, call 1-877-835-5472.
BREKIYA® is a registered trademark of Amneal Pharmaceuticals LLC.
Distributed by:
Amneal Specialty, a division of Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Manufactured by:
Amneal Pharmaceuticals Pvt. Ltd.
Ahmedabad 382213, INDIA
Rev. 05-2025-01
Medication Guide
|
BREKIYA®[breh-kee-yah] (dihydroergotamine mesylate) injection, for subcutaneous use |
|||
|
What is the most important information I should know about BREKIYA? BREKIYA can cause serious side effects, including:
|
|||
Do not take medicines known as strong CYP3A4 inhibitors, such as: |
|||
|
|
|
|
|
|
| |
| These are not all of the medicines that could affect how BREKIYA works. Your healthcare provider can tell you if it is safe to take BREKIYA with other medicines. | |||
|
What is BREKIYA? BREKIYA is a prescription medicine used for the acute treatment of migraine with or without aura and acute cluster headaches in adults.
|
|||
|
Who should not take BREKIYA? Do not use BREKIYA if you:
|
|||
|
|
|
|
|
|
|
|
|
| ||
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take BREKIYA with other medicines. |
|||
|
Before you take BREKIYA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your healthcare provider will decide if you can take BREKIYA with your other medicines. Especially tell your healthcare provider if you take: |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
| These are not all of the medicines that could affect how BREKIYA works. Your healthcare provider can tell you if it is safe to take BREKIYA with other medicines. | |||
|
How should I use BREKIYA?
|
|||
|
What are the possible side effects of BREKIYA? BREKIYA can cause serious side effects, including: See “What is the most important information I should know about BREKIYA?”
|
|||
|
|
|
|
|
|
|
|
| |||
The most common but serious side effects of BREKIYA are heart problems that happen but may lead to death. These heart problems include:
Symptoms of these heart problems include: See “heart attack and other heart problems.”
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of BREKIYA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
|
How should I store BREKIYA?
|
|||
|
General information about the safe and effective use of BREKIYA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BREKIYA for a condition for which it was not prescribed. Do not give BREKIYA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BREKIYA that is written for health professionals. |
|||
|
What are the ingredients in BREKIYA? Active ingredient: dihydroergotamine mesylate, USP
Inactive ingredients: ethanol, glycerin, water for injection, methanesulfonic acid or sodium hydroxide. Amneal Specialty, a division of Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Amneal Pharmaceuticals Pvt. Ltd. Ahmedabad 382213, INDIA
|
|||
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 05/2025
INSTRUCTIONS FOR USE
 |  |
|
This Instructions for Use contains information on how to inject BREKIYA. If there are any questions concerning the use of BREKIYA autoinjector, ask your healthcare provider, pharmacist or visit www.brekiya.com. |
|
| Important Information You Need to Know Before Injecting BREKIYA | |
|
Warnings
|
|
|
Dosing
|
|
| Storing BREKIYA Autoinjector | |
Keep BREKIYA and all medicines out of the reach of children. |
|
| Autoinjector Parts | |
|
This autoinjector is a prefilled single dose (1 time use), disposable device that gives a 1 mg subcutaneous (under the skin) injection of BREKIYA. Figure A shows a new and used autoinjector. 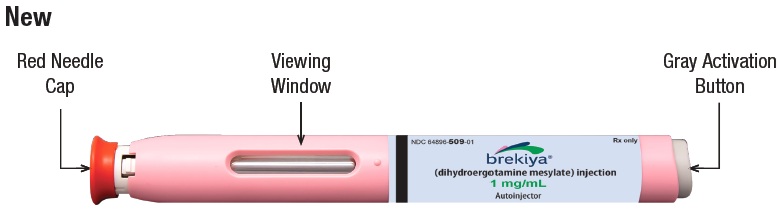 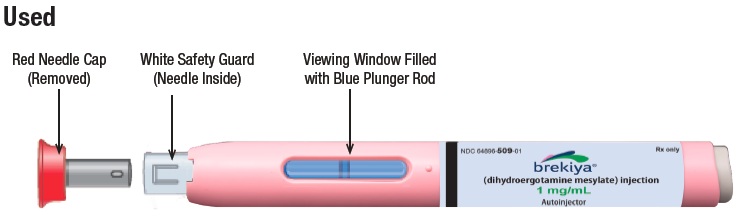
Figure A |
|
| Preparing to Inject BREKIYA | |
| Step 1: Wash Hands and Gather Supplies | |
|
1.1 Before you begin, wash your hands with soap and water (See Figure B). |  Figure B |
|
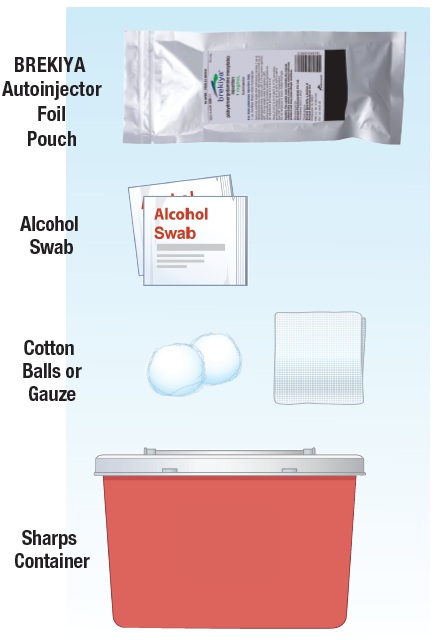
1.2 Gather the following supplies (See Figure C):
|
Figure C |
| Step 2: Remove and Inspect the Autoinjector | |
|
2.1 Tear open the foil pouch from the notch to remove the autoinjector (See Figure D). |  Figure D |
|
2.2 Check the expiration date printed on the autoinjector (See Figure E). Do not use the autoinjector if the expiration date has passed. 2.3 Check the liquid medicine in BREKIYA by looking through the viewing window (See Figure E). The liquid medicine in BREKIYA should be clear and colorless. It is normal to see one or more air bubbles. Do not inject if the liquid medicine appears cloudy, discolored, or has floating flakes or particles. Do not inject if the autoinjector appears to be damaged or broken. | 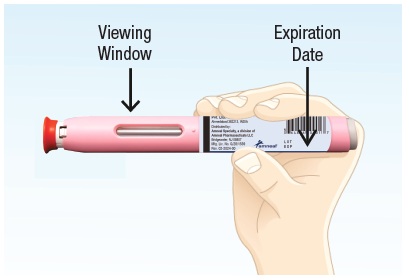 Figure E |
| Step 3: Select and Prepare Injection Site | |
|
3.1 Locate your injection site. BREKIYA autoinjector should be injected into the skin in the middle of your thigh (See Figure F). Do not inject into moles, scars, birthmarks, or areas where the skin is tender, bruised, red, or hard. Do not inject through clothing. Do not inject into the same spot 2 times in a row. Important: This injection should be at least 2 inches away from the last injection site. | 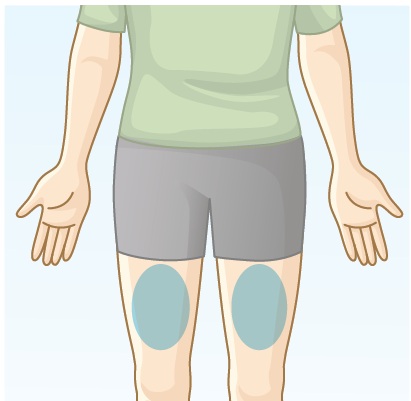 Figure F |
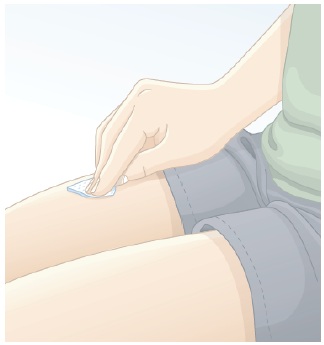
|
3.2 Clean the injection site with an alcohol swab (See Figure G). Wait for the injection site to air dry before giving the injection. Do not touch the clean injection site again before you inject BREKIYA. |  Figure G |
| Injecting BREKIYA | |
| Step 4: Remove Red Needle Cap and Position the Autoinjector | |
|
4.1 Remove the red needle cap by pulling it straight off (See Figure H) and throw it away in the household trash.
Do not bend or twist the cap while removing.
Do not touch the white safety guard after the red needle cap is removed. | 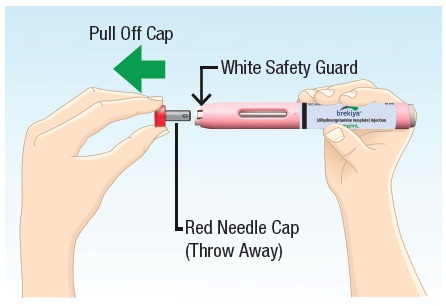 Figure H |
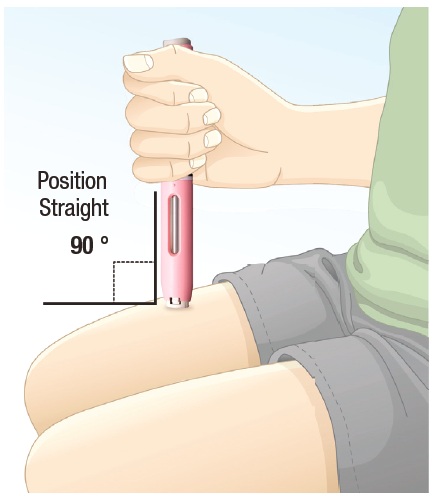
|
4.2 Position the autoinjector straight onto the cleaned injection site with the white safety guard resting on the skin (See Figure I). |
Figure I |
| Step 5: Give Injection | |
|
5.1 Push the autoinjector down against the skin until you do not see the white safety guard (See Figure J). Important: Keep downward pressure on the skin throughout the injection process. | 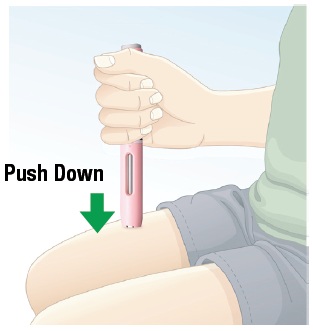 Figure J |
|
5.2 Press and release the gray activation button to start the injection (See Figure K). You will hear a “click” sound when the injection starts. Be Patient. Do not lift the autoinjector after the first click. Important: If you do not hear a “click” when you press the gray activation button, make sure that the autoinjector is pressed down against the skin, then try again. | 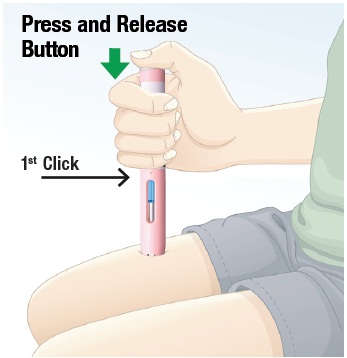 Figure K |
|
5.3 Hold the autoinjector down against the skin for at least 10 seconds to make sure you get the full dose of BREKIYA (See Figure L). Note: You may hear a second “click”, which means you are close to the end of the injection. Whether or not you hear a second “click” you must hold down the autoinjector against your skin for at least 10 seconds. Do not lift up the autoinjector early. | 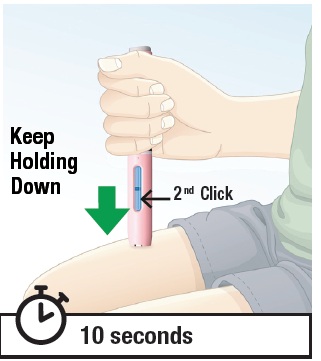 Figure L |
|
5.4 Make sure the injection is finished by looking at the viewing window. The viewing window will turn completely blue (See Figure M) when the full dose of BREKIYA has been given.
Note: Even if the inspection window is not completely blue, do not try to use the autoinjector again. | 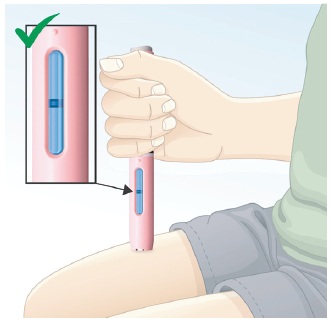 Figure M |
| Throwing Away (Disposing of) BREKIYA Autoinjector | |
| Step 6: Lift and Throw away (dispose of) | |
|
6.1 Lift the autoinjector straight up, away from the skin. The white safety guard will drop down and lock over the needle (See Figure N). Note: If you think that you have not received the full dose, do not repeat the injection using a new autoinjector. Call your healthcare provider right away for assistance. | 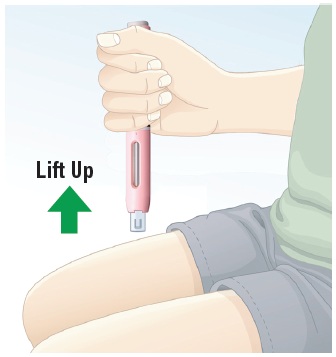 Figure N |
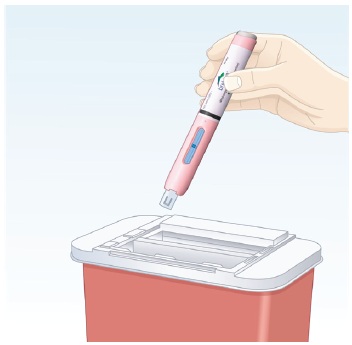
|
6.2 Throw away (dispose of) the used autoinjector into a FDA-cleared sharps disposal container (See Figure O). Do not throw away (dispose of) the autoinjector into household trash. |
Figure O |
| 6.3 Treat the injection site as needed (See Figure P). It is normal to see a drop of blood or medicine at the injection site. It will not affect your dose. |
Figure P |
| Additional Information on how to Throw Away (Dispose of) BREKIYA | |
Distributed by: Amneal Specialty, a division of Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Amneal Pharmaceuticals Pvt. Ltd. Ahmedabad 382213, INDIA |
|
This Instructions for Use have been approved by the U.S. Food and Drug Administration Issued: 05/2025
| BREKIYA
dihydroergotamine mesylate injection |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Amneal Pharmaceuticals LLC (123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amneal Pharmaceuticals Private Limited | 860156658 | analysis(64896-509) , manufacture(64896-509) , pack(64896-509) , sterilize(64896-509) | |
Frequently asked questions
More about Brekiya (dihydroergotamine)
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antimigraine agents
- Breastfeeding