Bacitracin Injection: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection, powder, for solution
Drug class: Miscellaneous antibiotics
Medically reviewed by Drugs.com. Last updated on Dec 12, 2024.
On This Page
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bacitracin and other antibacterial drugs, Bacitracin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
For Intramuscular Use
WARNING
Nephrotoxicity
Bacitracin in parenteral (intramuscular) therapy may cause renal failure due to tubular and glomerular necrosis. Its use should be restricted to infants with staphylococcal pneumonia and empyema when due to organisms shown to be susceptible to bacitracin. It should be used only where adequate laboratory facilities are available and when constant supervision of the patient is possible.
Renal function should be carefully determined prior to and daily during therapy. The recommended daily dose should not be exceeded and fluid intake and urinary output maintained at proper levels to avoid kidney toxicity. If renal toxicity occurs the drug should be discontinued. The concurrent use of other nephrotoxic drugs, particularly streptomycin, kanamycin, polymyxin B, polymyxin E (colistin), and neomycin should be avoided.
Bacitracin Injection Description
Sterile Bacitracin, USP is an antibiotic for intramuscular administration. Bacitracin is derived from cultures of Bacillus subtilis (Tracey). It is a white to pale buff, hygroscopic powder, odorless or having a slight odor. It is freely soluble in water; insoluble in acetone, chloroform, and ether. While soluble in alcohol, methanol, and glacial acetic acid, there is some insoluble residue. It is precipitated from its solutions and inactivated by many of the heavy metals.
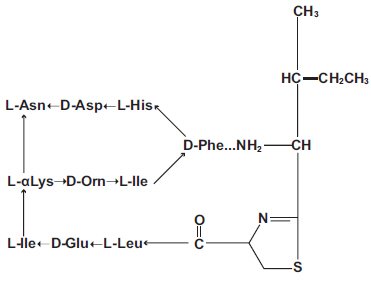
The structural formula is:
|
|
|
| bacitracin A | |
The molecular formula is: C66H103N17O16S. Bacitracin is comprised of a polypeptide complex and Bacitracin A is the major component in this complex. The molecular weight of Bacitracin A is 1422.71.
Bacitracin Injection - Clinical Pharmacology
Bacitracin exerts pronounced antibacterial action in vitro against a variety of gram-positive and a few gram-negative organisms. However, among systemic diseases, only staphylococcal infections qualify for consideration of bacitracin therapy. Bacitracin is assayed against a standard and its activity is expressed in units, 1 mg having a potency of not less than 50 units.
Absorption of bacitracin following intramuscular injection is rapid and complete. A dose of 200 or 300 units/kg every 6 hours gives serum levels of 0.2 to 2 mcg/mL in individuals with normal renal function. The drug is excreted slowly by glomerular filtration. It is widely distributed in all body organs and is demonstrable in ascitic and pleural fluids after intramuscular injection.
Indications and Usage for Bacitracin Injection
In accordance with the statements in the "Warning Box" the use of intramuscular bacitracin is limited to the treatment of infants with pneumonia and empyema caused by staphylococci shown to be susceptible to the drug.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Bacitracin and other antibacterial drugs, Bacitracin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
This drug is contraindicated in those individuals with a history of previous hypersensitivity or toxic reaction to it.
Precautions
See "Warning Box" for precautions in regard to kidney toxicity associated with intramuscular use of bacitracin.
Adequate fluid intake should be maintained orally, or if necessary, by parenteral method.
As with other antibiotics, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be instituted.
Prescribing Bacitracin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for patients
Patients should be counseled that antibacterial drugs including Bacitracin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Bacitracin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Bacitracin or other antibacterial drugs in the future.
Adverse Reactions/Side Effects
Related/similar drugs
Bacitracin Injection Dosage and Administration
TO BE ADMINISTERED INTRAMUSCULARLY ONLY
Infant dose
For infants under 2500 grams-900 units/kg/24 hours in 2 or 3 divided doses. For infants over 2500 grams-1,000 units/kg/24 hours, in 2 or 3 divided doses. Intramuscular injections of the solution should be given in the upper outer quadrant of the buttocks, alternating right and left and avoiding multiple injections in the same region because of the transient pain following injection.
Preparation of Solutions
Should be dissolved in sodium chloride injection containing 2 percent procaine hydrochloride. The concentration of the antibiotic in the solution should not be less than 5,000 units per mL nor more than 10,000 units per mL.
Diluents containing parabens should not be used to reconstitute bacitracin; cloudy solutions and precipitate formation have occurred.
Reconstitution of the 50,000 unit vial with 9.8 mL of diluent will result in a concentration of 5,000 units per mL.
Solutions are stable for one week when stored in a refrigerator 2˚ to 8˚C (36˚ to 46˚F).
How is Bacitracin Injection supplied
Sterile Bacitracin, USP is available as a pack of ten (10's) with each vial containing 50,000 units (NDC 72572-025-10).
| BACITRACIN
bacitracin injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Civica, Inc. (081373942) |
| Registrant - Xellia Pharmaceuticals ApS (305814345) |
More about bacitracin
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous antibiotics
- Breastfeeding
- En español