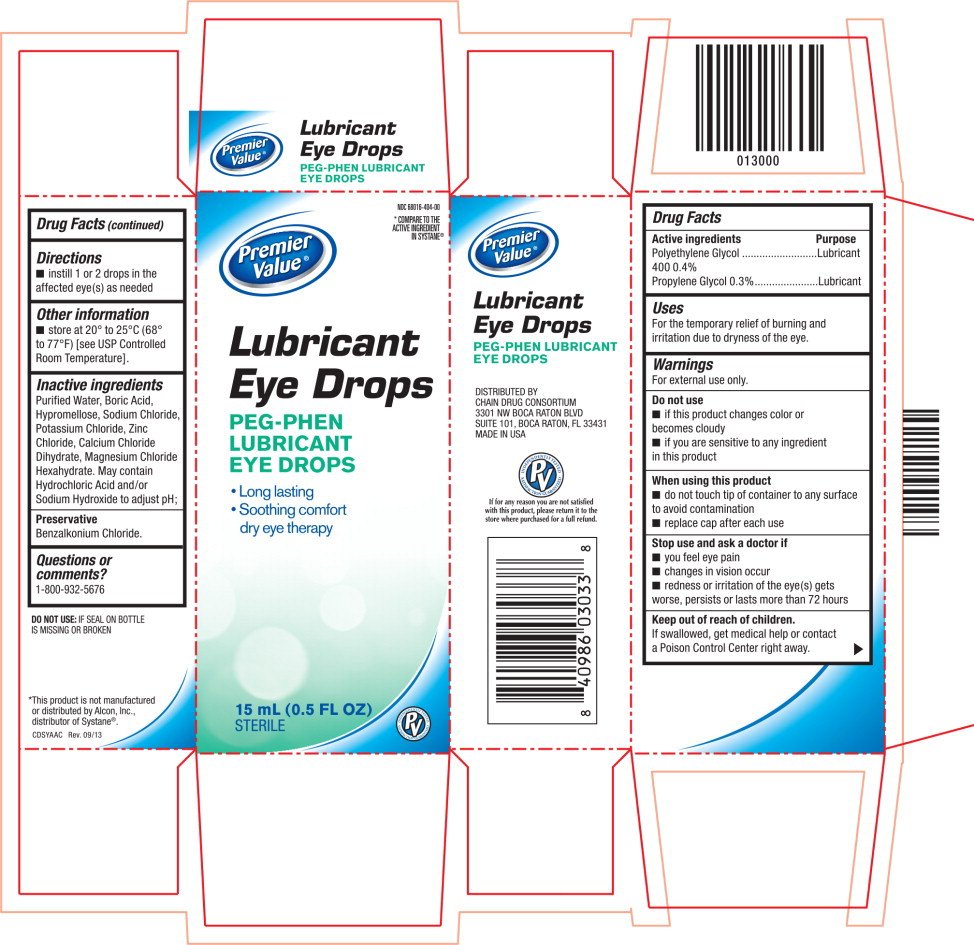
Lubricant Eye Drops
Dosage form: solution/ drops
Ingredients: Polyethylene Glycol 400 4mg in 1mL, Propylene Glycol 3mg in 1mL
Labeler: Chain Drug Consortium
NDC code: 68016-404
Medically reviewed by Drugs.com. Last updated on Oct 14, 2024.
Drug Facts
Polyethylene Glycol 400 0.4%
Propylene Glycol 0.3%
Lubricant
Lubricant
For the temporary relief of burning and irritation due to dryness of the eye.
For external use only.
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye(s) gets worse, persists or lasts more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
- instill 1 or 2 drops in the affected eye(s) as needed
- store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Purified Water, Boric Acid, Hypromellose, Sodium Chloride, Potassium Chloride, Zinc Chloride, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate. May contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH;
Benzalkonium Chloride.
1-800-932-5676
Principal Display Panel Text for Container Label:
68016-404-00
Premier
Value® Logo
Lubricant
Eye Drops
PEG-PHEN
LUBRICANT
EYE DROPS
- Long lasting
- Soothing comfort dry eye therapy
15 mL (0.5 FL OZ) STERILE

Principal Display Panel Text for Carton Label:
68016-404-00
*COMPARE TO THE
ACTIVE INGREDIENT
IN SYSTANE®
Premier
Value ® Logo
Lubricant
Eye Drops
PEG-PHEN
LUBRICANT
EYE DROPS
- Long lasting
- Soothing comfort
dry eye therapy
15 mL (0.5 FL OZ)
STERILE PV Brand Logo
| LUBRICANT EYE DROPS
polyethylene glycol 400 and propylene glycol solution/ drops |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
| Registrant - Akorn, Inc. (062649876) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Akorn, Inc | 603980319 | MANUFACTURE(68016-404), ANALYSIS(68016-404), STERILIZE(68016-404), PACK(68016-404), LABEL(68016-404) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
