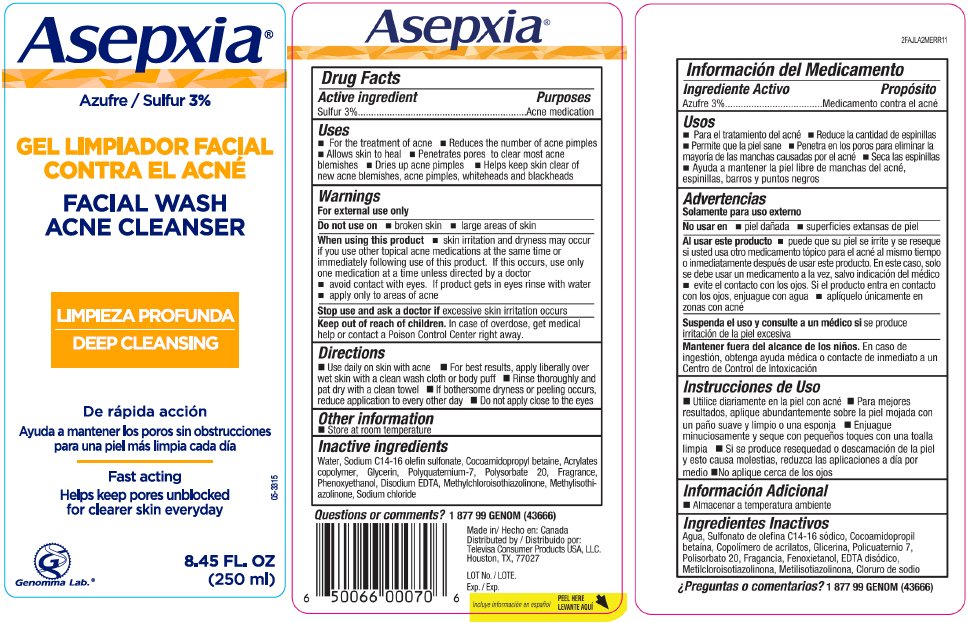
Asepxia Facial Wash Acne Cleanser Acne Medication
Dosage form: lotion
Ingredients: Sulfur 30mg in 1mL
Labeler: Genomma Lab USA Inc.
NDC code: 50066-051
Medically reviewed by Drugs.com. Last updated on Nov 18, 2024.
Acne Medication
Drug Facts
Sulfur 3%
Acne Medication
- ♦
- For the treatment of Acne
- ♦
- Reduces the number of acne pimples
- ♦
- Allows skin to heal
- ♦
- Penetrates pores to clear most acne blemishes
- ♦
- Dries up acne pimples
- ♦
- Helps keep skin clear of new acne blemishes, acne pimples, whiteheads and blackheads
For external use only
- ♦
- broken skin
- ♦
- large areas of skin
- ♦
- skin irritation and dryness may occur if you use other topical acne medications at the same time or immediately following use of this product. If this occurs, use only one medication at a time unless directed by a doctor
- ♦
- avoid contact with eyes. If product gets in eyes rinse with water
- ♦
- apply only to areas of acne
Stop use and ask a doctor if excessive skin irritation occurs
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- ♦
- Use daily on skin with acne
- ♦
- For best results, apply liberally over wet skin with a clean wash cloth or body puff, concentrating on areas prone to acne
- ♦
- Rinse thoroughly and pat dry with a clean towel
- ♦
- If bothersome dryness or peeling occurs, reduce application to every other day
- ♦
- Do not apply close to the eyes
- ♦
- Store at room temperature
Water, Sodium C14-16 olefin sulfonate, Cocamidopropyl betaine, Acrylates copolymer, Glycerin, Polyquaternium-7, Polysorbate 20, Fragrance, Phenoxyethanol, Disodium EDTA, Methylchloroisothiazolinone, Methylisothiazolinone, Sodium Chloride
1 877 99 GENOM (43666)
Distributed by:
Televisa Consumer Products USA, LLC.
Houston, TX, 77027
Asepxia®
Sulfur 3%
FACIAL WASH
ACNE CLEANSER
DEEP CLEANSING
Fast acting
Helps keep pores unblocked
for clearer skin everyday
05-3315
Genomma Lab.®
8.45 FL. OZ
(250 ml)
| ASEPXIA FACIAL WASH ACNE CLEANSER
ACNE MEDICATION
sulfur lotion |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Genomma Lab USA Inc. (832323534) |
| Registrant - Garcoa, Inc. (036464697) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Garcoa, Inc. | 036464697 | MANUFACTURE(50066-051) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
