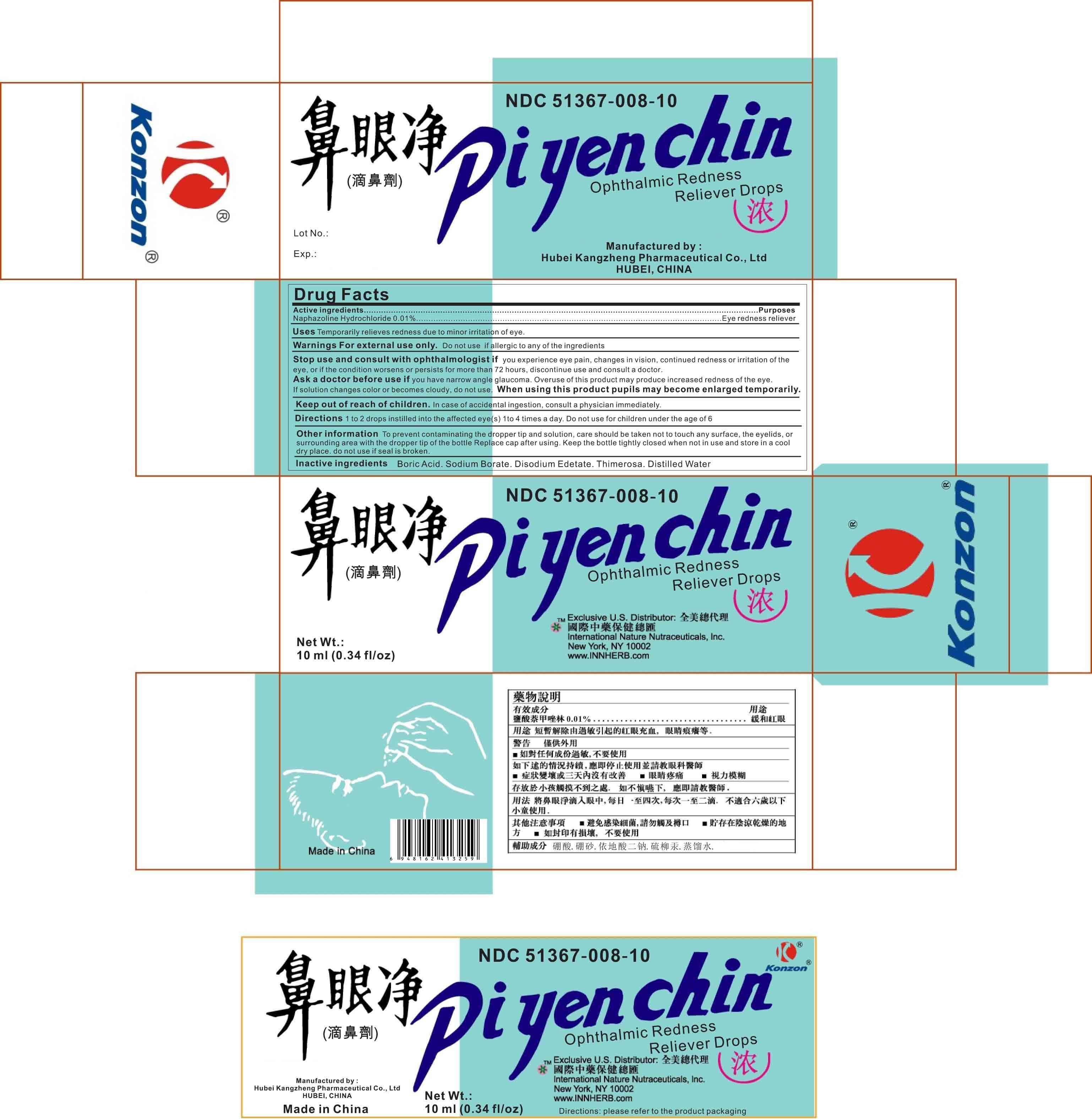
Pi Yen Chin
Dosage form: solution/ drops
Ingredients: NAPHAZOLINE HYDROCHLORIDE 1mg
Labeler: International Nature Nutraceuticals
NDC code: 51367-008
Medically reviewed by Drugs.com. Last updated on Jul 17, 2025.
Naphazoline Hydrochloride: 0.01%
Purpose: Eye redness reliever
Temporarily relieves redness due to minor irritation of eye
Do not use if allergic to any of the ingredients.
If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor.
Ask a doctor before use if you have narrow angle glaucoma.
Overuse of this product may produce increased redness of the eye.
If solution changes color or becomes cloudy, do not use.
When using this product pupils may become enlarged temporarily
1 to 2 drops instilled into the affected eye(s) 1 to 4 times a day. Do not use for children under the age of 6.
To prevent contaminating the dropper tip and solution, care should be taken not to touch any surface, the eyelids, or surronding area with the dropper tip of the bottle. Replace cap after using. Keep bottle tightly closed when not in use and store in a cool dry place. Do not use if seal is broken.
Boric Acid, Sodium Borate, Disodium Edetate, Thimerosal, Distilled Water
| PI YEN CHIN
naphazoline hydrochloride solution/ drops |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - International Nature Nutraceuticals (006106879) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Hubei Kangzheng Pharmaceutical Co., Ltd | 530031426 | manufacture(51367-008) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
