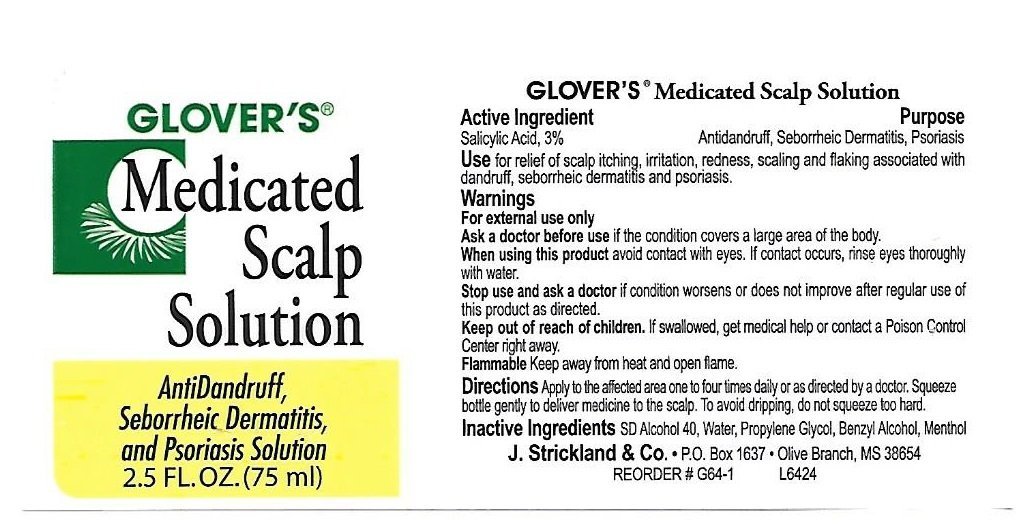
Glovers Medicated Anti-Dandruff Scalp
Dosage form: solution
Ingredients: SALICYLIC ACID 30mg in 1mL
Labeler: J. Strickland & Co.
NDC code: 12022-011
Medically reviewed by Drugs.com. Last updated on Oct 28, 2024.
Salicylic Acid, 3%
Antidandruff,
Seborrheic Dermatitis,
Psoriasis
for relief of scalp itching, irritation, redness, scaling and flaking associated with dandruff, seborrheic dermatitis and psoriasis.
For External Use Only.
the condition covers a large area of the body
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
condition worsens or does not improve after regular use of this product as directed.
If swallowed, get medical help or call a poison control center right away.
Keep away from heat and open flame.
- Apply to the affected area one to four times daily or as directed by a doctor
- Use after shampooing or between shampoos.
SD Alcohol 40, Water, Propylene Glycol, Benzyl Alcohol, Menthol.

| GLOVERS MEDICATED ANTI-DANDRUFF SCALP
salicylic acid solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - J. Strickland & Co. (007023112) |
| Registrant - J. Strickland & Co. (007023112) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| J. Strickland & Co. | 007023112 | manufacture(12022-011), pack(12022-011), label(12022-011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
