Glovers Dandruff Control Medicine, Regular
Dosage form: suspension
Ingredients: SULFUR 25mg in 1mL
Labeler: J. Strickland & Co.
NDC code: 12022-007
Medically reviewed by Drugs.com. Last updated on Oct 28, 2024.
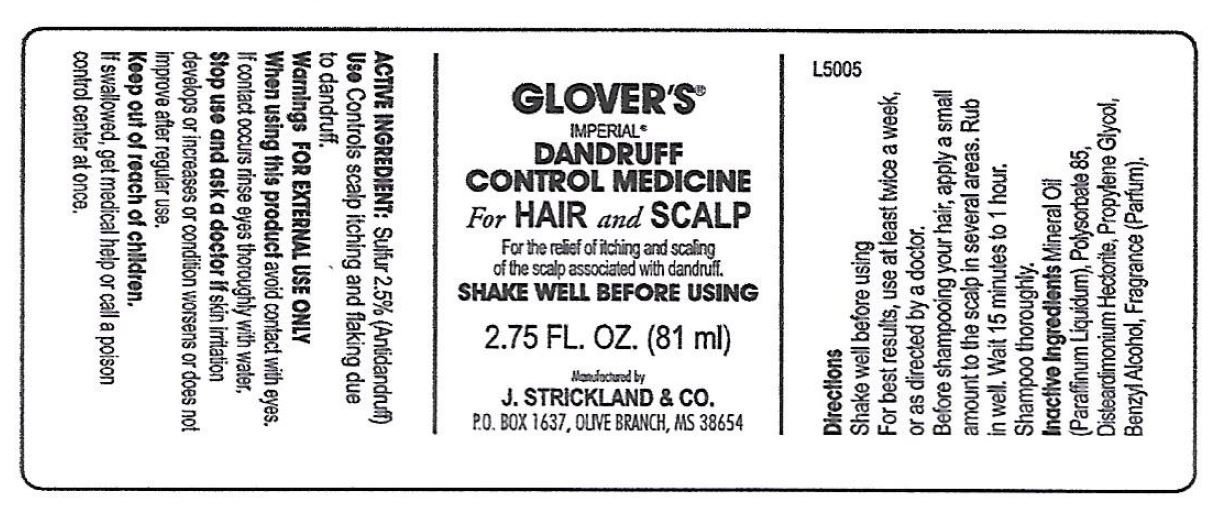
Active Ingredients:
Sulfur, 2.5%
Purpose
Antidandruff
Uses:
Controls scalp itching and flaking due to dandruff
Warnings:
For External Use Only.
When using this product
- do not get into eyes. If contact occurs rinse eyes thoroughly with water.
Stop use and consult a doctor if
- if skin irritation develops or increases.
- condition worsens or does not improve after regular use.
Keep out of reach of children
If swallowed, get medical help or call a poison control center at once.
Directions:
- Shake well before using.
- For best results, use at leats twice a week, or as directed by a doctor.
- Before shampooimg your hair, apply a small amount to the scalp in several areas. Rub in well. Wait 15 minutes to 1 hour Shampoo thoroughly
Inactive Ingredients:
Mineral Oil (Paraffum Liquidum), Polysorbate-85, Disteardimonium Hectorite, Propylene Glycol, Benzyl Alcohol, Fragrance (Parfum).
Package Labeling Bottle
Package Labeling:
| GLOVERS DANDRUFF CONTROL MEDICINE, REGULAR
sulfur suspension |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - J. Strickland & Co. (007023112) |
| Registrant - J. Strickland & Co. (007023112) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| J. Strickland & Co. | 007023112 | manufacture(12022-007), pack(12022-007), label(12022-007) | |
Document Id: 96b265f6-1a5a-5834-e053-2a95a90a0a2c
Set id: 18700036-11ec-4370-bcc6-b9e2c732e7d9
Version: 3
J. Strickland & Co.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
