barr 200mg 588 Pill: green & white, capsule/oblong, 18mm
The pill with imprint barr 200mg 588 (Green & White, Capsule/Oblong, 18mm) has been identified as Didanosine 200 mg and is used for HIV Infection, and Nonoccupational Exposure. It belongs to the drug class nucleoside reverse transcriptase inhibitors (NRTIs) and is not a controlled substance.
Images for barr 200mg 588


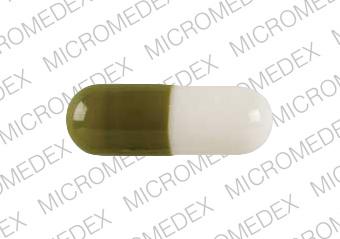
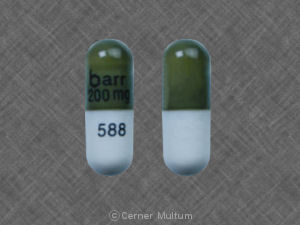
Didanosine
- Imprint
- barr 200mg 588
- Strength
- 200 mg
- Color
- Green & White
- Size
- 18.00 mm
- Shape
- Capsule/Oblong
- Availability
- Prescription only
- Drug Class
- Nucleoside reverse transcriptase inhibitors (NRTIs)
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Teva Pharmaceuticals USA
- National Drug Code (NDC)
- 00555-0588 (Discontinued)
- Inactive Ingredients
-
ferrosoferric oxide,
croscarmellose sodium,
D&C Yellow No. 10,
FD&C Blue No. 1,
FD&C Blue No. 2,
indigotindisulfonate sodium,
FD&C Red No. 40,
hypromellose 2910 (3 mPa.s),
hypromellose 2910 (6 mPa.s),
hypromellose 2910 (50 mPa.s),
methacrylic acid - ethyl acrylate copolymer (1:1) type a,
microcrystalline cellulose,
polydextrose,
polyethylene glycol 8000,
propylene glycol,
shellac,
silicon dioxide,
sodium hydroxide,
magnesium silicate,
titanium dioxide,
triacetin,
triethyl citrate,
D&C Red No. 33,
FD&C Yellow No. 6
Note: Inactive ingredients may vary.
See also:
More about didanosine
- Check interactions
- Compare alternatives
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: nucleoside reverse transcriptase inhibitors (NRTIs)
- Breastfeeding
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
