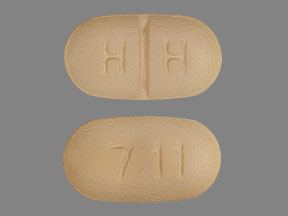
711 Pill: brown, oval, 10mm
The pill with imprint 711 (Brown, Oval, 10mm) has been identified as Mesalamine Delayed-Release 1.2 g and is used for Ulcerative Colitis, Maintenance, Crohn's Disease, Maintenance, Ulcerative Colitis, Active, Ulcerative Proctitis, and Inflammatory Bowel Disease. It belongs to the drug class 5-aminosalicylates and is not a controlled substance.
Images for 711


Mesalamine Delayed-Release
- Imprint
- 711
- Strength
- 1.2 g
- Color
- Brown
- Size
- 10.00 mm
- Shape
- Oval
- Availability
- Prescription only
- Drug Class
- 5-aminosalicylates
- Pregnancy Category
- B - No proven risk in humans - excluding Asacol and Asacol HD, C - Risk cannot be ruled out - Asacol and Asacol HD
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- Zydus Pharmaceuticals (USA) Inc.
- National Drug Code (NDC)
- 68382-0711
- Inactive Ingredients
-
carboxymethylcellulose sodium,
microcrystalline cellulose,
ferric oxide red,
ferric oxide yellow,
hypromelloses,
magnesium stearate,
methacrylic acid,
silicon dioxide,
sodium starch glycolate type A potato,
magnesium silicate,
titanium dioxide,
triethyl citrate
Note: Inactive ingredients may vary.
Related images for "711"
More about mesalamine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (426)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: 5-aminosalicylates
- Breastfeeding
Patient resources
- Mesalamine drug information
- Mesalamine rectal
- Mesalamine (Oral) (Advanced Reading)
- Mesalamine (Rectal) (Advanced Reading)
Other brands
Lialda, Pentasa, Apriso, Asacol HD, ... +4 more
Professional resources
- Mesalamine monograph
- Mesalamine (FDA)
- Mesalamine Capsules (FDA)
- Mesalamine Controlled-Release Capsules (FDA)
- Mesalamine Delayed Release Tablets (FDA)
Other brands
Lialda, Pentasa, Apriso, Asacol, ... +5 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.