Hyqvia Dosage
Generic name: HUMAN IMMUNOGLOBULIN G 100mg in 1mL; HYALURONIDASE (HUMAN RECOMBINANT) 160[USP'U] in 1mL
Dosage form: subcutaneous injection
Drug class: Immune globulins
Medically reviewed by Drugs.com. Last updated on Jul 29, 2025.
Recommended Dosage
For subcutaneous use only
The recommended Recombinant Human Hyaluronidase (rHuPH20) dose is 80 U/g Immune Globulin (IgG), which corresponds to 0.5 mL rHuPH20 solution per 10 mL Immune Globulin Infusion (Human), 10% solution for both indications.
The recommended Immune Globulin Infusion (Human), 10% dose is given below.
Primary Immunodeficiency (PI)
Initiation of Treatment with HYQVIA
- For patients previously on another IgG treatment, administer the first dose approximately one week after the last infusion of their previous treatment.
- Gradually increase the dose and frequency from a 1-week dose to a 3-or 4-week dose (see ramp-up schedule in Table 1).
- Initiating treatment at a full monthly dose was not evaluated in the clinical trial.
| Week | Infusion Number | Dose Interval | Example for 30 grams per 4 weeks |
|---|---|---|---|
| 1 | 1st infusion | 1-week-dose | 7.5 grams |
| 2 | 2nd infusion | 2-week-dose | 15 grams |
| 3 | No infusion | Not Applicable (NA) | NA |
| 4 | 3rd infusion | 3-week-dose | 22.5 grams |
| 5 | No infusion | NA | NA |
| 6 | No infusion | NA | NA |
| 7 | 4th infusion (if required) | 4-week-dose | 30 grams |
For patients switching from Immune Globulin Intravenous (Human) [IGIV] treatment:
- Administer HYQVIA at the same dose and frequency as the previous intravenous treatment, after the initial dose ramp-up.
For patients at risk for measles exposure:
- If a patient has been exposed to measles, administer a dose of HYQVIA as soon as possible and within 6 days of exposure. A dose of 400 mg/kg should provide a serum level >240 mIU/mL of measles antibodies for at least two weeks.
- If a patient is at risk of future measles exposure and receives a dose of less than 530 mg/kg every 3 to 4 weeks, the dose should be increased to at least 530 mg/kg. This should provide a serum level of 240 mIU/mL of measles antibodies for at least 22 days after infusion.
Individualization of Dose
- If HYQVIA is administered at the same dose and frequency, the serum IgG levels from HYQVIA should be comparable to serum IgG levels from intravenous treatment.
For dose adjustment:
- Calculate the difference between the patient's serum IgG trough level during HYQVIA treatment and the IgG trough level during the previous intravenous treatment.
- Find this difference (in mg/dL) in the columns of Table 2 and the corresponding amount (in mL) by which to increase or decrease the dose based on the patient's body weight and desired change in IgG trough level.
| Body Weight | 100 mg/dL | 200 mg/dL | 300 mg/dL | 400 mg/dL |
|---|---|---|---|---|
|
||||
| 10 kg | 3 mL | 6 mL | 9 mL | 12 mL |
| 20 kg | 6 mL | 12 mL | 18 mL | 24 mL |
| 30 kg | 9 mL | 18 mL | 27 mL | 36 mL |
| 40 kg | 12 mL | 24 mL | 36 mL | 48 mL |
| 50 kg | 15 mL | 30 mL | 45 mL | 61 mL |
| 60 kg | 18 mL | 36 mL | 55 mL | 73 mL |
| 70 kg | 21 mL | 42 mL | 64 mL | 85 mL |
| 80 kg | 24 mL | 48 mL | 73 mL | 97 mL |
| 90 kg | 27 mL | 55 mL | 82 mL | 109 mL |
| 100 kg | 30 mL | 61 mL | 91 mL | 121 mL |
| 110 kg | 33 mL | 67 mL | 100 mL | 133 mL |
| 120 kg | 36 mL | 73 mL | 109 mL | 145 mL |
| 130 kg | 39 mL | 79 mL | 118 mL | 158 mL |
| 140 kg | 42 mL | 85 mL | 127 mL | 170 mL |
Example 1: A patient with a body weight of 80 kg has a measured IgG trough level of 800 mg/dL and the reference trough level is 1000 mg/dL. The trough level difference is 200 mg/dL (1000 mg/dL minus 800 mg/dL). The dose of HYQVIA would be increased by 48 mL (4.8 grams) per dosing interval.
Example 2: A patient with a body weight of 60 kg has a measured IgG trough level of 1000 mg/dL and the reference trough level is 900 mg/dL. The trough level difference is -100 mg/dL (900 mg/dL minus 1000 mg/dL). The dose of HYQVIA would be decreased by 18 mL (1.8 grams) per dosing interval.
HYQVIA can be used to administer a full therapeutic dose in one site up to every four weeks. Adjust the frequency and number of infusion sites taking into consideration volume, total infusion time, and tolerability. Adjust the frequency as needed so that the patient receives the same weekly equivalent dose.
Example 3: When adjusting a dose of 30 grams administered every 3 weeks, administer 40 grams of HYQVIA every 4 weeks. If a higher trough level is required relative to intravenous treatment at 3- or 4-week intervals, increase the dose or decrease the dosing interval. Evaluate the use of a second site or infusing at shorter intervals when the volume of HYQVIA is greater than 600 mL.
If a patient misses a dose, administer the missed dose as soon as possible and then resume scheduled treatments as applicable.
If HYQVIA is administered at a different interval than the previous treatment, either intravenously or subcutaneously, then Table 2 should not be used and the dose of HYQVIA should be adjusted, if necessary, based on clinical response.
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
General instruction:
- Before initiating therapy with HYQVIA, calculate the weekly equivalent dose to plan for the ramp-up schedule. Dose and dosing frequency can be adjusted based on the individual clinical response.
- A dose ramp-up schedule is recommended by gradually increasing the SC infusion volume until the full dose is reached to ensure the patients’ tolerability.
- Depending on the treating physician's discretion, in patients who tolerate the first two infusions well, subsequent infusions may be administered by gradually increasing doses and decreasing dose intervals, considering the volume and total infusion time.
- Doses less than or equal to 0.4 g/kg may be administered without a ramp-up provided acceptable patient tolerance.
For patients switching from Immune Globulin Intravenous (Human) [IGIV] treatment:
- Patients switching from intravenous administration of immune globulin must be on stable1 doses of IGIV.
- Before initiating therapy with HYQVIA, calculate the weekly equivalent dose by dividing the last IGIV dose by the IGIV dose interval in weeks.
- The starting dose and dosing frequency of HYQVIA is the same as the patient’s previous IGIV treatment. The typical dosing interval range in the clinical trial for HYQVIA was 4 weeks. For patients with less frequent IGIV dosing (greater than 4 weeks), the dosing interval can be converted to 3 or 4 weeks while maintaining the same monthly equivalent IgG dose.
- Administer the calculated one-week dose (1st infusion) two weeks after the last IGIV infusion as directed in section 2.3. One week after the first HYQVIA dose, administer another weekly equivalent dose (2nd infusion).
- A ramp-up period can take up to 9 weeks (Table 3), depending on the dosing interval and tolerability.
- 1
- Variations in the dosing interval of up to ±7 days or monthly equivalent dose amount of up to ±20% between the subject’s IgG infusions are considered a stable dose.
| Week* | Infusion Number | Dose Interval | Example for 100 g every 4 weeks |
|---|---|---|---|
|
|||
| 1 | No infusion | Not applicable (NA) | NA |
| 2 | 1st infusion | 1-week-dose | 25g |
| 3 | 2nd infusion | 1-week-dose | 25g |
| 4 | 3rd infusion | 2-week-dose | 50g |
| 5 | No infusion | NA | NA |
| 6 | 4th infusion | 3-week-dose | 75g |
| 7 | No infusion | NA | NA |
| 8 | No infusion | NA | NA |
| 9 | 5th infusion | 4-week-dose | 100g (Full dose reached) |
Preparation and Handling
- Visually inspect both vials of HYQVIA for discoloration and particulate matter prior to administration.
- The appearance of the Immune Globulin Infusion (Human), 10% of HYQVIA can vary from clear or slightly opalescent and colorless or pale yellow.
- The appearance of the rHuPH20 of HYQVIA should be clear and colorless.
- Do not use either component of HYQVIA if either solution is cloudy or has particulates.
- Allow refrigerated product to come to room temperature before use. Do not apply heat or place in microwave.
- Do not shake HYQVIA.
- Do not mix the rHuPH20 and the Immune Globulin Infusion (Human), 10% of HYQVIA into the same container prior to administration.
- Do not mix or administer components of HYQVIA with other products. Administer components of HYQVIA sequentially. Do not use either component alone.
- Flush the infusion line with normal saline or Dextrose 5% in water (D5W) if required.
- HYQVIA contains no preservative. Discard any unused product according to local standards for biohazard products.
Administration
HYQVIA should be administered by a healthcare professional, caregiver or self-administered by the patient after appropriate training.
- Infusion of Immune Globulin Infusion (Human), 10% requires an infusion pump capable of infusing a patient’s therapeutic dose at infusion rates up to 300 mL/hr/site. The pump must have the ability to titrate the flow rate up or down if required to improve tolerability. To ensure maximum flow rates, use a subcutaneous needle set that is 24 gauge and labeled for high flow rates.
- Infusion site leakage can occur during or after subcutaneous administration of immunoglobulin, including HYQVIA. Consider using longer needles (14 or 12 mm rather than 9 mm) and/or more than one infusion site.
- Infuse the two components of HYQVIA sequentially, beginning with the rHuPH20. If using two or three infusion sites, divide the rHuPH20 evenly between all sites.
- Initiate infusion of the full dose of the Immune Globulin Infusion (Human), 10% through the same subcutaneous needle set within approximately 10 minutes of the rHuPH20 infusion. For each full or partial vial of Immune Globulin Infusion (Human), 10% used, administer the entire contents of the rHuPH20 vial.
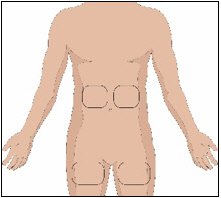
Selection of Infusion Site(s)
The suggested site(s) for the infusion of HYQVIA are the abdomen and thighs. If two sites are used, the two infusion sites should be on opposite sides of the body. If using three infusion sites, the sites should be at least 10 cm apart. Avoid bony prominences, or areas that are scarred, inflamed, or infected.
Volume per Site
Primary immunodeficiency (PI)
Administer up to 600 mL per site for patients whose body weight is greater than or equal to 40 kg and up to 300 mL per site for patients whose body weight is less than 40 kg.
A second site can be used at the discretion of the physician and patient based on tolerability and total volume. If a second site is used, administer half the total volume of rHuPH20 of HYQVIA in each site.
Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP)
On a given infusion day, the maximum infusion volume should not exceed 1200 mL for patients weighing ≥40 kg or 600 mL for patients weighing <40 kg. If the maximum daily dose limit is exceeded or the patient cannot tolerate the infusion volume, the dose may be administered over multiple days in divided doses with 48 to 72 hours between doses to allow absorption of infusion fluid at the infusion site(s). The dose can be administered at 1, 2, or 3 infusion sites with a maximum infusion volume of 600 mL per site (or as tolerated). If using three sites, the maximum is 400 mL per site.
Rate of Infusion
Primary immunodeficiency (PI)
Administer the rHuPH20 of HYQVIA at an initial rate per site of approximately 1 to 2 mL per minute, or as tolerated.
Administer Immune Globulin Infusion (Human), 10% of HYQVIA at rates as shown in Table 4 for the initial infusions. If the patient tolerates these infusions at the full dose and maximum rate, adjust both the time intervals and number of rate changes of the ramp-up used for successive infusions at the discretion of the physician and patient.
| First 2 Infusions | Subsequent 2 or 3 Infusions | |||
|---|---|---|---|---|
| Patients <40 kg (<88lbs) |
Patients ≥40 kg (≥88lbs) |
Patients <40 kg (<88lbs) |
Patients ≥40 kg (≥88lbs) |
|
| Intervals | Rate per site | Rate per site | Rate per site | Rate per site |
| Minutes | mL per hour | mL per hour | mL per hour | mL per hour |
| 5 - 15 | 5 | 10 | 10 | 10 |
| 5 - 15 | 10 | 30 | 20 | 30 |
| 5 - 15 | 20 | 60 | 40 | 120 |
| 5 - 15 | 40 | 120 | 80 | 240 |
| Remainder of infusion | 80 | 240 | 160 | 300 |
Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP)
The full dose of rHuPH20 solution is infused at a rate of 1 to 2 mL per minute (60 mL to 120 mL/hr) per infusion site or as tolerated. Immune Globulin Infusion, (Human), 10% can be administered through the same subcutaneous needle set within approximately 10 minutes after the rHuPH20 infusion is completed.
Patients with body weight of 40 kg or above
The Immune Globulin Infusion (Human), 10% should be infused at an initial rate of 10 mL per hour per infusion site. If tolerated, the rate of the administration may be increased at intervals of 5 to 15 minutes and to a maximum infusion rate of 240 mL per hour per infusion site for the initial one or two infusions. For subsequent infusions the rate can be adjusted to a maximum of 300 mL per hour per infusion site.
Patients with body weight under 40 kg
The Immune Globulin Infusion, (Human), 10% should be infused at an initial rate of 5 mL per hour per infusion site. If well tolerated, the rate of the administration may be increased at intervals of 5 to 15 minutes and to a maximum infusion rate of 80 mL per hour per infusion site for the initial one or two infusions. For subsequent infusions the rate can be adjusted to a maximum of 160 mL per hour per infusion site.
Instructions for Administration
To administer HYQVIA using the FDA-cleared Vial Access Devices (VAD), refer to the Instructions For Use (IFU) provided with the VAD. Note: Use the VAD to administer HYQVIA in adult patients only.
To administer HYQVIA using a needle or needle-less transfer device, refer to the following instructions below.
Use aseptic technique when preparing and administering HYQVIA for infusion.
For more detail on steps, see accompanying Information for Patients.
1. Inspect the vials: Inspect for clarity, color, and expiration date(s).
2. Prepare for infusion:
- Gather supplies: HYQVIA dual vial unit(s), ancillary supplies, sharps container and infusion pump (program pump per physician recommendation following manufacturer’s instructions).
- Prepare a clean work area.
- Wash hands.
- If the Immune Globulin Infusion (Human), 10% and rHuPH20 are pooled into separate containers, skip to Step 5.
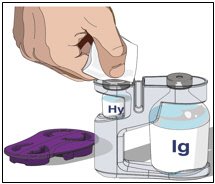
3. Prepare the rHuPH20 of HYQVIA (Labeled as “HY”):
- Remove the purple protective cap and ensure the blue vial caps are removed.
- Wipe each stopper with a sterile alcohol wipe and allow to dry.
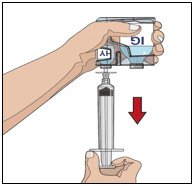
- Attach a syringe to a needle/needle-less transfer device. A sterile needle or needle-less transfer device [(18-22) gauge sterile needle] may be used for all vial sizes. Position the sharp tip of the needle/needle-less transfer device over the center of the vial stopper and insert it at a 90-degree angle. Inject air and then draw the full contents of each vial labeled “HY” into a single syringe, if possible.
- Attach the syringe containing the rHuPH20 to the subcutaneous needle set and prime up to the needle hub.
|
|
|
4. Prepare Immune Globulin Infusion (Human), 10% of HYQVIA (Labeled as “IG”):
- Wipe each stopper with a sterile alcohol wipe and allow to dry.
- Transfer the vial(s) labeled “IG” using one of the following methods:
- Pool into an infusion bag, using a transfer device per manufacturer’s directions. Attach and prime the pump administrating tubing; or
- Directly spike the vial using a vented pump administration tubing and prime as directed.
If using a syringe driver, transfer into the syringe(s), preferably using a vented spike.
|
|
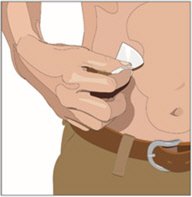
5. Prepare the infusion site(s):
- Potential sites for infusion include the middle to upper abdomen and thigh.
- Avoid: bony prominences, blood vessels, scars, or areas that are inflamed or infected.
- If two sites are desired, a bifurcated needle set may be used on opposite sides of the body.
- Rotate sites by choosing opposite sides of the body between successive infusions.
- Cleanse the infusion site(s) with a sterile alcohol wipe beginning at the center of each infusion site and moving outward in a circular motion. Allow the infusion site(s) to dry.
|
|
|
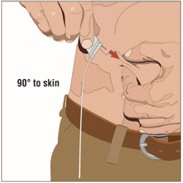
6. Insert and secure the 24-gauge subcutaneous needle:
- Pinch at least one inch of skin between two fingers. Insert the needle at a 90-degree angle into the subcutaneous tissue and secure the needle with sterile tape and check the placement.
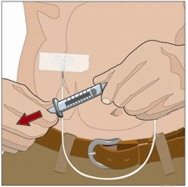
- If using the push method:
- Gently pull back on the plunger of the attached syringe and monitor for any blood return in the tubing.
- If blood is seen in the tubing, remove and discard the needle and repeat steps 3, 5, and 6 with a new subcutaneous needle and infusion site.
- If using the pump method:
- Close the clamp above the lower port of the pump tubing.
- Clean the lower port with an alcohol swab and allow to dry, for at least 30 seconds.
- Attach a 5 mL syringe to the lower port of the pump tubing.
- Pull back gently on the syringe plunger. If no blood, remove the syringe and open the lower port clamp.
- Secure the needle in place with a sterile protective dressing.
|
|
7. Administer the rHuPH20 of HYQVIA:
- If using push method:
- Infuse the rHuPH20manually at an initial rate of approximately 1 to 2 mL per minute per infusion site and increase as tolerated.
- If using a pump method:
- start at an initial rate at 60 mL to 120 mL/hr per infusion site and may increase as tolerated up to 300 mL/hr.
- If more than one site is used, divide the contents equally between sites. At the end of infusion, disconnect the empty syringe and attach the pump tubing/syringe containing the Immune Globulin Infusion (Human), 10% of HYQVIA to the same subcutaneous needle set.
8. Administer the Immune Globulin Infusion (Human), 10% of HYQVIA:
Within approximately 10 minutes of completing the infusion of the rHuPH20 of HYQVIA, start the variable rate program of the infusion pump to initiate the infusion of the full therapeutic dose of Immune Globulin Infusion (Human), 10% of HYQVIA. At the end of infusion, flush the infusion tubing up to the needle with normal saline or Dextrose 5% in water (D5W), if required.
9. Remove subcutaneous needle(s) from the infusion site(s):
After the infusion is complete, remove the needle set and cover with a protective dressing. Discard any partially used vial(s) and disposable supplies in accordance with local requirements.
10. Document the infusion:
Remove the peel-off label from each Immune Globulin Infusion (Human), 10% vial of HYQVIA used and affix to the patient’s treatment record or infusion log. In addition, record the time, date, dose, infusion site location and any reactions after each infusion.
For self-administration, provide the patient with instructions and training for infusion in the home or other appropriate setting.
More about Hyqvia (hyaluronidase / immune globulin)
- Check interactions
- Compare alternatives
- Side effects
- During pregnancy
- FDA approval history
- Drug class: immune globulins
- En español
Patient resources
Professional resources
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.