Abecma
Pronunciation: uh-BEK-muh
Generic name: idecabtagene vicleucel
Dosage form: suspension for intravenous infusion
Drug class: Miscellaneous antineoplastics
What is Abecma?
Abecma (idecabtagene vicleucel) is a CAR T-cell therapy used for treating relapsed or refractory multiple myeloma in certain adults. Abecma is a personalized one-time infusion made from a patient’s own white blood cells, which are genetically modified to recognize and attack multiple myeloma cells.
Abecma FDA approval was granted for treating relapsed or refractory multiple myeloma (rrMM) in adults who have received at least two prior therapies, including an immunomodulatory agent, proteasome inhibitor, and an anti-CD38 monoclonal antibody.
Abecma's FDA approval was based on positive results from the clinical trial KarMMa-3 Study Abecma patients had a success rate of:
- 72% overall response rate (ORR)
- 28% achieved a stringent complete response
- 25% had a very good partial response
- 19% achieved a partial response
These results highlight Abecma’s effectiveness in delaying disease progression in pre-treated multiple myeloma patients.
How Does Abecma Work?
Abecma is a personalized immunotherapy designed to enhance the body's immune response against multiple myeloma. It utilizes genetically engineered autologous T cells to target and eliminate cancer cells. The process works as follows:
- Genetic Modification: The T cells are engineered in a laboratory to express a chimeric antigen receptor (CAR), which specifically recognizes B-cell maturation antigen (BCMA), a protein found on multiple myeloma cells.
- CAR Activation & Targeting: Once infused back into the patient, these modified T cells seek out and bind to BCMA-expressing myeloma cells.
- Cancer Cell Destruction: Upon binding, the CAR T cells become activated, triggering an immune response that leads to the destruction of multiple myeloma cells through cytotoxic mechanisms.
This targeted approach enhances the immune system’s ability to attack multiple myeloma cells, potentially leading to prolonged remission for patients who have exhausted other treatment options.
What is the Abecma treatment process?
- Leukapheresis: White blood cells are collected.
- Manufacturing Process: The infusion is created in a specialized lab (takes about 4 weeks).
- Pre-Infusion Chemotherapy: Patients receive three days of chemotherapy before Abecma infusion.
- Infusion: Abecma infusion is administered two days after the completion of lymphodepleting chemotherapy. It is given as an intravenous infusion through a catheter (tube) placed into your vein.Premedication is given 30 to 60 minutes before infusion.
- Monitoring: Patients must remain at the treatment center for at least 7 days post-infusion and within 2 hours of the facility for 4 weeks for safety monitoring.
Your infusion may be delayed for up to 7 days if you have any of the following conditions:
- unresolved serious adverse events (especially pulmonary events, cardiac events, or hypotension), including those after preceding chemotherapies,
- active infections or inflammatory disorders.
Abecma side effects
Common Abecma side effects
Common Abecma side effects may include:
- cytokine release syndrome - CRS (confusion, trouble breathing, fast or irregular heartbeats, feeling light-headed or very tired);
- headache, dizziness;
- problems with speech;
- low blood cell counts;
- fever, chills, tiredness, or other signs of infection;
- decreased appetite, severe nausea or diarrhea;
- pain in your bones, joints, or muscles;
- swelling anywhere in your body; or
- cold symptoms such as stuffy nose, sneezing, sore throat, cough.
Serious Abecma side effects:
Get emergency medical help if you have signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
A serious side effect of Abecma is called cytokine release syndrome (CRS). Tell your caregivers right away if you have signs of this condition: fever, chills, trouble breathing, severe vomiting or diarrhea, tremors, shaking, fast or irregular heartbeats, feeling light-headed, or feeling very tired. Your caregivers will have medication available to quickly treat CRS if it occurs.
Also, tell your caregivers or seek emergency medical attention if you have signs of nerve problems, blood disorders, or infection. Symptoms may include problems with speech, problems with thinking or memory, confusion, fatigue, fever, swelling, or a seizure.
Call your doctor at once if you have:
- headaches, dizziness, drowsiness;
- problems with thinking or memory;
- trouble speaking or understanding what is said to you;
- tremors, anxiety, sleep problems;
- seizure;
- right-sided upper stomach pain, vomiting, loss of appetite, yellowing of your skin or eyes, and not feeling well; or
- low blood cell counts - fever, chills, tiredness, flu-like symptoms, mouth sores, skin sores, easy bruising, unusual bleeding, pale skin, cold hands and feet, feeling light-headed or short of breath.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Related/similar drugs
Warnings
Cytokine release syndrome or CRS. Abecma can cause a very common side effect called cytokine release syndrome, which can be severe or fatal. Symptoms of CRS include fever, difficulty breathing, dizziness or light-headedness, nausea, headache, fast heartbeat, low blood pressure, or fatigue. Tell your healthcare provider right away if you develop fever or any of these other symptoms.
Infections. Abecma can increase the risk of life-threatening infections that may lead to death. Tell your healthcare provider right away if you develop fever, chills, or any signs or symptoms of an infection. It may also lower one or more types of blood cells (red blood cells, white blood cells, or platelets), which may make you feel weak or tired or increase your risk of severe infection or bleeding. After treatment, your healthcare provider will test your blood to check for this. Tell your healthcare provider right away if you get a fever, are feeling tired, or have bruising or bleeding.
Other cancer risk. Abecma may increase your risk of getting cancer including certain types of blood cancers, called T-cell malignancies. Your healthcare provider should monitor you for these.
Nerve problems. Abecma may also cause life-threatening nerve problems, blood disorders, or other life-threatening reactions. Tell your caregivers or seek emergency medical attention if you have problems with speech, problems with thinking or memory, confusion, seizures, fatigue, shortness of breath, or chest pain.
Having Abecma in your blood may cause a false-positive human immunodeficiency virus (HIV) test result by some commercial tests.
It is important that you tell your healthcare providers that you have received Abecma and to show them your Patient Wallet Card. Your healthcare provider may give you other medicines to treat your side effects.
Before taking this medicine
Tell your doctor if you have ever had:
- an active infection or inflammation;
- hepatitis B;
- cytomegalovirus; or
- if you have received a vaccine in the past 6 weeks.
Pregnancy
Abecma is not recommended for women who are pregnant, and pregnancy after Abecma infusion should be discussed with the treating physician. Women may need to take a pregnancy test before receiving this medicine. You may also need to use birth control to prevent pregnancy during and shortly after treatment with Abecma and chemotherapy.
Assess immunoglobulin levels in newborns of mothers treated with Abecma. If you receive Abecma during pregnancy, your baby's blood may need to be tested after it is born. This is to evaluate any effects the medicine may have had on the baby.
Breastfeeding
It may not be safe to breastfeed while using this medicine. Ask your doctor about any risks.
What should I avoid while using Abecma?
Abecma can cause weakness, drowsiness, confusion, problems with memory or coordination, and seizures. Avoid driving or operating machinery for at least 2 weeks after you are treated with Abecma.
Vaccination with live virus vaccines is not recommended for at least 6 weeks before the start of lymphodepleting chemotherapy, during Abecma treatment, and until immune recovery following your infusion.
Do not donate blood, an organ, or any tissues or cells.
What other drugs will affect Abecma?
Other drugs may interact with Abecma, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Abecma Package Insert
Review the Abecma Package Insert for more detailed information about this medicine. The Abecma Package Insert contains more comprehensive information on Indications and Usage, Dosage and Administration, Clinical Pharmacology, Clinical Studies, Drug Interaction, and more. Discuss any medical questions you have with your doctor or other health care provider. This is not all the information you need to know about this medicine for safe and effective use, and it does not take the place of talking to your doctor about your treatment.
The Package Insert is sometimes called Abecma Prescribing Information (PI) or the FDA label.
Storage
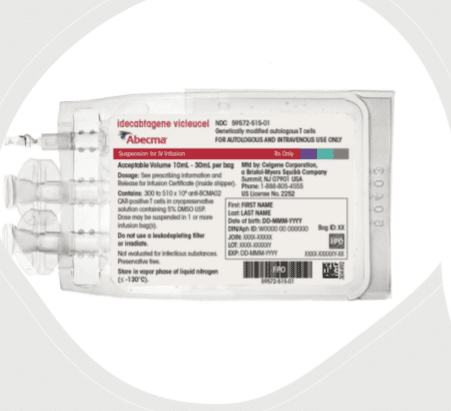
- Store Abecma frozen in the vapor phase of liquid nitrogen (less than or equal to minus 130°C).
- Thaw Abecma prior to the infusion, according to the Abecma Prescribing Information.
Ingredients
Abecma is supplied in one or more infusion bag(s) containing a frozen suspension of genetically modified autologous T cells in 5% DMSO.
Abecma is made specifically for each individual patient with their own white blood cells. Match the identity of the patient with the patient identifiers on the cassette(s) and infusion bag(s) upon receipt.
Abecma Manufacturer
Abecma Manufacturer Celgene Corporation, a Bristol-Myers Squibb Company, 556 Morris Avenue, Summit, NJ 07901.
Marketed by: Celgene Corporation, a Bristol-Myers Squibb Company (Summit, NJ 07901), and bluebird bio, Inc. (Cambridge, MA 02142).
Abecma Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There is 1 for Abecma.
Abecma (idecabtagene vicleucel) - Celgene Corporation, a Bristol-Myers Squibb Company
| Formulation type | Strength |
|---|---|
| Bag | 300 to 460 X 10^6 CHIMERIC ANTIGEN RECEPTOR (CAR)-POSITIVE T CELLS |
| Bag | 300 to 510 X 10^6 CHIMERIC ANTIGEN RECEPTOR (CAR)-POSITIVE T CELLS |
Popular FAQ
What is CAR T-cell therapy and how does it work?
CAR T-cell therapy is a personalized treatment that uses a patient's own immune cells to fight certain cancers and autoimmune diseases. By harnessing and reprogramming a patient’s own immune cells, this therapy offers a new option for those who have not responded to conventional treatments.
Continue readingIs Abecma an orphan drug?
Abecma is considered an orphan drug because it is used to treat multiple myeloma, a rare disease. Orphan drugs are used to treat, prevent or diagnose rare “orphan” diseases that affect fewer than 200,000 people in the U.S.
Continue readingReferences
More about Abecma (idecabtagene vicleucel)
- Check interactions
- Compare alternatives
- Drug images
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous antineoplastics
- En español
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.