Zelnate
This treatment applies to the following species:See productdata.aphis.usda.gov for a summary of the studies approved by the USDA for licensing this product. The package insert may also contain additional information developed by the licensee.
DNA Immunostimulant
For Intramuscular or Intranasal Administration to Cattle
FOR VETERINARY USE ONLY
02337
READ IN FULL
Description
The innate immune system in cattle has been shown to provide a potent, rapid, nonspecific, protective response to infectious agents, such as Mannheimia haemolytica that can lead to Bovine Respiratory Disease (BRD). BRD is a serious condition that commonly causes lung lesions, reduced lung capacity and mortality.
ZELNATE® is a bacterial-produced plasmid DNA with a liposome carrier that stimulates the innate immune system and has been shown to be effective against bovine respiratory disease due to Mannheimia haemolytica.
The freeze-dried (desiccate) product is packaged with two different sterile diluents. The First Sterile Rehydrator (vial 1) is used to reconstitute the desiccate cake (vial 2), and then transferred to the Final Sterile Solution (vial 3) to achieve the proper concentration for administration.
INDICATION
This product has been shown to be effective for the treatment of cattle, 4 months of age or older, against bovine respiratory disease due to Mannheimia haemolytica. For more information regarding efficacy and safety data, see productdata.aphis.usda.gov.
This product has been shown to be effective at the time of, or within 24 hours after, a perceived stressful event.
IMPORTANT STORAGE CONDITIONS
Store Refrigerated
2°C to 8°C (35°F to 46°F)
DO NOT FREEZE.
Stability has been demonstrated for at least 8 hours after reconstitution if vial is refrigerated and sterility is maintained.
Individual Study Summary - Study# 200270
|
Study Type |
Efficacy |
|||||
|
Pertaining to |
Mannheimia haemolytica |
|||||
|
Study Purpose |
Efficacy against bovine respiratory disease |
|||||
|
Product Administration |
One dose administered by IM route at the time of challenge. Control group administered diluent only |
|||||
|
Study Animals |
64 Holstein steers of 3-4 months of age; randomized into 2 groups of 32 calves each |
|||||
|
Challenge Description |
live M. haemolytica inoculum |
|||||
|
Interval observed after challenge |
Observed daily for 5 days. Lungs were evaluated 5 days after challenge. |
|||||
|
Results |
The percent of lung mass that was abnormal (consolidated) was calculated/scored for every animal. For animals that died prior to Day 5, the necropsy lung score was not included in the analysis. 5 number summary for lung consolidation |
|||||
|
Treatment |
Minimum |
Q1 |
Median |
Q3 |
Maximum |
|
|
Controls |
0% |
6% |
10% |
15% |
33% |
|
|
Treated |
0% |
1% |
4% |
10% |
22% |
|
|
Raw data shown on the table below. The animals that died prior to Day 5 are marked with an asterick (*). The deaths prior to Day 5 were: 1/32 in Treated group: 1/32 in Control group. Diagnosis was severe bovine respiratory disease for calf in Control group. |
||||||
|
USDA Aproval Date |
28-Feb-2013 |
|||||
Lung consolidation scores (%), in order to rank:
|
Treated |
0% |
0% |
1% |
1% |
1% |
1% |
1% |
1% |
2% |
2% |
3% |
3% |
3%* |
4% |
4% |
4% |
|
Control |
0% |
0% |
3% |
3% |
3% |
4% |
6% |
6% |
6% |
7% |
7% |
7% |
8% |
8% |
10% |
10% |
|
Treated (Cont.) |
4% |
5% |
5% |
6% |
8% |
9% |
10% |
10% |
10% |
11% |
12% |
13% |
13% |
15% |
18% |
22% |
|
Control (Cont.) |
10% |
10% |
10% |
11% |
13% |
14% |
15% |
15% |
18% |
18% |
21% |
23% |
27% |
29% |
33% |
34%* |
* death prior to Day 5
METHOD OF ADMINISTRATION
Inject 2 mL intramuscularly at the time of, or within 24 hours after, a perceived stressful event (for example: weaning, shipping, commingling or adverse environmental conditions). Alternatively, spray 2 mL into one nostril using an atomization tip attached to the syringe; the atomizer should produce a fine mist of particles 30-100 microns in size for delivery to the mucosal membranes.
Zelnate Caution
In case of human exposure, contact a contact a physician. Use entire contents when first opened. Inactivate unused contents before disposal.
PRECAUTION
Do not administer within 21 days of slaughter. Do not mix with other products, except as specified on the label. This product has not been tested in pregnant animals.
OTHER INFORMATION
Contains no antibiotics and no preservatives.
HOW SUPPLIED
Vials of 10 and 50 doses.
Mixing process must be completed in the appropriate order. Transfer needle must be fully inserted to prevent spillage.
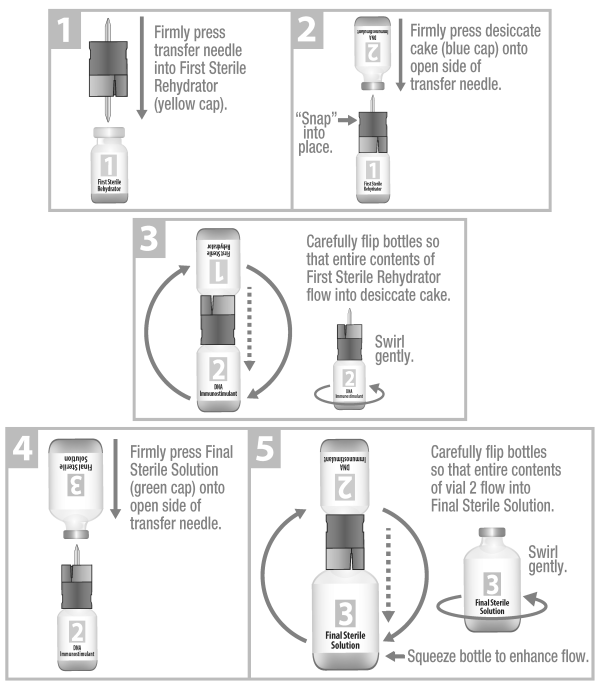
ZELNATE® is ready for use.

MANUFACTURED BY:
Diamond Animal Health, Inc., Des Moines, IA 50327
U.S. Veterinary License No. 213
PCN 9381.D0.
Made in U.S.A.
December, 2020
LV2012
DISTRIBUTED BY:
Elanco US Inc., Greenfield, IN 46140
1-800-633-3796
This product is based on technology developed by Juvaris BioTherapeutics and is patent protected. Animal health applications are being developed exclusively under the rights of Elanco and are protected by patents.
90198129
W1a
CPN: 1131202.0
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
