Witness Lepto
This treatment applies to the following species:CANINE LEPTOSPIRA ANTIBODY TEST KIT
I. General Information
WITNESS™ Lepto should be used in sick dogs with symptoms compatible with Canine Leptospirosis: signs of renal and/or hepatic failure, lethargy, anorexia, vomitus, acute febrile illness, pulmonary hemorrhage, anemia, uveitis, and abortion. WITNESS™ Lepto detects IgM antibodies against Leptospira serovars Canicola, Pomona, Icterohaemorrhagiae, and Grippotyphosa in the dog’s whole blood, plasma, or serum. The test is simple, requires minimal equipment and capabilities, and results are obtained in 10 minutes. A positive result on WITNESS™ Lepto associated with clinical signs is strongly indicative of acute Leptospirosis. WITNESS™ Lepto cannot differentiate between antibodies to pathogenic and nonpathogenic serovars (Autumnalis and Bratislava). IgM antibodies resulting from vaccination may be detected.
Ii. Test Principles
WITNESS™ Lepto is a simple test based on Rapid Immuno Migration (RIM™) technology that detects the presence of anti-Leptospira IgM antibody in canine whole blood, serum or plasma. Sensitized particles form a complex with the Leptospira antibody present in the sample. The formed complex migrates along a membrane. The complex is then captured on a sensitized reaction zone and when anti-Leptospira IgM is present at high enough levels, accumulation of the complex in this zone causes the formation of a visible pink/red band (2). A pink/red band in the control window (3) ensures that the test was performed correctly.
III. SAMPLE COLLECTION
● The test can be performed on whole blood (anticoagulated with EDTA), serum, or plasma.
● Always collect samples with a sterile needle and syringe.
● Hemolysis does not significantly interfere with the test, but strongly hemolyzed specimens may partly obscure a weak positive band.
WARNING! Leptospira may be present in the sample and is infectious to humans. The sample, test, and all materials exposed to the sample are biohazardous and should be disposed of accordingly. Wear gloves when handling sample and performing test.
IV. SAMPLE STORAGE
Anticoagulated whole blood samples should preferably be tested immediately after collection but not longer than 4 hours after collection, if stored at room temperature.
If testing is further delayed, samples should be kept refrigerated (whole blood samples up to 24 hours and serum or plasma up to 7 days at 2 °C to 7 °C; 35 °F to 45 °F).
For long term storage, samples (serum or plasma only) should be kept frozen (-20 °C; -4 °F or colder).
V. KIT CONTENTS
● 5 Pouches, each containing 1 test device and desiccant
● 1 Saline buffer bottle (5 mL)
● 5 Self-filling capillary pipettes
● Instructions for use
VI. PRECAUTIONS
● Do not use this kit or any of its components after the expiration date.
● Kit should be stored at 2 °C - 25 °C (35 °F - 77 °F). Kit should not be frozen.
● Use the test immediately after the pouch is first opened (within 10 minutes).
● Avoid touching or damaging the membrane in the sample well or the results window.
● The WITNESS™ device should be placed on a flat, horizontal surface while performing the test.
● Use a separate pipette for each sample.
● Ensure that the capillary tube in the sample pipette has been completely filled (an air bubble will be visible at the top of the capillary tube if it has not been completely filled).
● Hold buffer bottle vertically when dispensing buffer.
● Handle all samples as biohazardous material.
● Saline buffer is preserved with sodium azide.
● For animal use only.
VII. TEST PROCEDURE
Important: This test uses a special self-filling pipette to accurately deliver a small sample volume (5 µl) to the test. While the pipette is different from what most users are familiar with, it is very accurate and easy to use. Follow the instructions carefully; failure to do so may result in improper function of the device.
Allow the buffer drops to fall onto the membrane at window #1. Do not touch buffer drops or buffer bottle tip directly to the membrane.
1. SAMPLE APPLICATION
● Tear open a pouch provided and place the test device on a flat horizontal surface.
● Gently grip the stem of the pipette. Do not squeeze the bulb! The pipette will fill automatically by capillary action when placed in the sample. The bulb is only squeezed when depositing the sample on the test.
● Hold a tube of sample and pipette at an angle to allow proper filling of pipette. Without squeezing, place the tip of the pipette in the sample. The capillary tube in the pipette will fill automatically in 2-3 seconds. Remove pipette from sample when the capillary tube has filled. If the capillary tube is not filling, hold the sample and pipette at a greater angle, or repeatedly remove and reinsert the pipette tip into the sample.
● Touch the tip of the pipette to the sample pad and carefully squeeze the bulb to express the sample, making sure fingers are covering the vent holes on the pipette bulb.

2. BUFFER DISPENSING
● Remove the cap from the buffer bottle, hold it vertically and add three drops of buffer to the test well.
3. READING TEST
After 10 minutes, observe the presence or absence of pink/red bands in the reading windows (2) and (3).
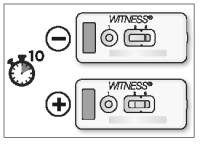
Note:
● It is possible to read the test before 10 minutes if two pink/red bands are clearly visible in reading windows (2) and (3).
● The presence of only one band in control window (3) prior to the end of the development time (10 minutes) does not mean that the test is complete, as a test band in window (2) may appear more slowly than the control band.
VIII. RESULTS
Valid Results
● Test is valid if a pink/red band is present in control window (3).
Interpretation of Results
● Positive: One band in reading window (2), with one band in window (3): sample is positive for anti-Leptospira IgM.
● Negative: No band in reading window (2), with one band in window (3): sample is negative for anti-Leptospira IgM.
● Invalid test: No band in control window (3).

Note:
● The presence of any pink/red band in reading window (2), even if weak, should be considered a positive test if the test was valid.
SYMBOL DESCRIPTIONS

Manufacturer
Delpharm Biotech, 2 rue Alexander Fleming, 69007 Lyon - FRANCE
Distributed by: Zoetis Inc., Kalamazoo, MI 49007, USA
VPN/PCN 190D/5052.20
1-888-963-8471
www.zoetis.com
K000320
April 2017
CPN: 3690620.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
