Vetscan Heartworm Antigen Test Kit
This treatment applies to the following species:I. INTENDED USE
The Vetscan® Heartworm Rapid Test is a visual and rapid test for the qualitative detection of Dirofilaria immitis (D. immitis) in canine or feline anticoagulated whole blood, serum or plasma. This test is for veterinary use only.
Dirofilaria immitis is a common filarial nematode of dogs, cats and wild canids. The disease is transmitted by mosquitoes and it has a world-wide distribution. The adult worms reside in the heart and the adjacent blood vessels. The parasite can interfere with the blood circulation, heart functions, and may damage other vital organs (1).
The Vetscan Heartworm Rapid Test is based on using heartworm-specific antibodies in an immuno-chromatographic sandwich assay. Antibody-coated colloidal gold particles bind to D. immitis antigen in the sample. The bound antigen flows through the strip and is then captured by antibodies on the test strip. The accumulation of the captured gold particle/ antigen complex causes a color to become visible in the Test line (T) area. To serve as a procedural control, a colored line in the Control line (C) area will always appear regardless if the sample is positive or negative.
II. INSTRUCTIONS FOR USE
● Samples must be at room temperature 15° to 27°C (59° to 80°F), before running the assay -DO NOT HEAT.
● Previously frozen or older samples must be centrifuged before use.
● Serum or Plasma, either fresh, previously frozen or stored at 2° to 7°C (36° to 45°F), may be used in this test. Serum or plasma may be stored for up to 7 days at 2° to 7°C. For longer storage, sample should be frozen (-20°C or colder).
● Whole Blood must be anticoagulated (e.g. EDTA, heparin) and may be used either fresh or after refrigeration at 2° to 7°C (36° to 45°F) for up to 5 days.
● Hemolyzed samples will not affect the results.
● EDTA or heparin in plasma will not affect the results.
III. PRECAUTIONS AND WARNINGS
● Important: Do not remove device from the pouch until ready for use.
● For veterinary use only.
● Do not use components after expiration date.
● Device must be used as soon as possible after removing from pouch.
● The device should be in a horizontal position on a flat surface while the test is performed.
● Use a separate transfer pipette for each test.
● All wastes should be properly decontaminated prior to disposal.
● The buffer reagent is not interchangeable from serial to serial.
● Handle samples as biohazardous materials
● Dispose of contents/container in accordance with local/regional/national/international regulations.
● Contains sodium azide as a preservative.
IV. STORAGE
● Devices and test reagents must be stored at room temperature 15° to 27°C (59° to 80°F).
● Devices and test reagents are stable until the expiration date when stored at 15° to 27°C (59° to 80°F).
V. KIT COMPONENTS
● Test Devices
● Chase Buffer Bottle (6mL each)
● Transfer Pipettes
● Instructions for Use
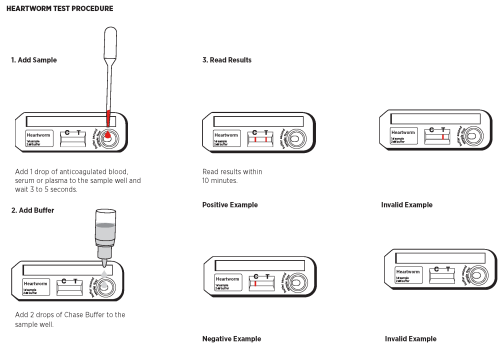
VI. TEST PROCEDURE
1. Remove the Test Device from the protective pouch and place on a flat surface. Label the Test Device with the subject I.D. or control identification.
2. Gently mix the sample by inverting.
3. Using the Transfer Pipette provided, transfer 1drop of sample (anticoagulated whole blood, serum, or plasma) into the sample well. Wait for the sample to be absorbed (3-5 seconds).
4. Holding the Chase Buffer Bottle vertically, promptly add 2 drops of the Chase Buffer to the sample well.
5. Read the results within 10 minutes. High positives may appear as soon as 1 minute, and low positive results may take up to 10 minutes to appear. Do not read results after 15 minutes. Colored lines which appear after 15 minutes are not diagnostic and should be ignored.
VII. INTERPRETATION OF TEST RESULTS
Positive Results
The test is positive if two colored lines appear. One colored line will appear at the Test line (T) area and other at the Control line (C) area. Any intensity of the Test line (T) area should be considered positive. Colored lines may be lighter or darker than each other.
Negative Results
The test is negative if only the Control line (C) appears.
Invalid Results
The test is invalid if no colored line appears at the Control line (C) area even if a colored line appears in the Test line (T) area. If no colored line appears at the Control line (C) within 10 minutes, add an additional drop of the sample and wait for 5 minutes. If a colored line still does not appear in the C area, the test is invalid and should be repeated. Colored lines which appear after 15 minutes are not diagnostic and should be ignored.
REFERENCE
1. Rawling, C.A., et al. (1982). Four types of Occult Dirofilaria immitis infection in dogs. Journal of the American Veterinary Medical Association 180:1323-1326.
Manufacturer
SA Scientific, 4919 Golden Quail, San Antonio, TX 78240 USA
VLN/PCN 373/5018.00
1-210-699-8800
Distributor
Zoetis Inc., 333 Portage St., Kalamazoo, MI 49007 USA
1-888-963-8471
www.zoetisus.com
Imported by
Zoetis Belgium S.A., Rue Laid Burniat 1, 1348 Louvain-La-Neuve Belgium
51592600
Presentation: 25 and 100 test kits.
CPN: 3690608.0
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
