Thyro-Tabs Canine (0.2 mg) (Canada)
This treatment applies to the following species: Company: Lloyd
Company: Lloyd
(Levothyroxine sodium tablets), USP
DIN 02231021 (0.1 mg), 02231022 (0.2 mg), 02231023 (0.3 mg), 02231024 (0.4 mg), 02231025 (0.5 mg), 02231026 (0.6 mg), 02231027 (0.7 mg), 02231028 (0.8 mg)
Veterinary Use Only
Description
Each tablet contains synthetic crystalline levothyroxine sodium USP.
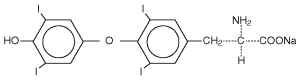
The structural formula for levothyroxine sodium is:
Indication: For use in dogs for correction of conditions associated with low circulating thyroid hormone (hypothyroidism).
Low serum circulating L-thyroxine (T-4) concentrations, coupled with clinical signs, are suggestive of hypothyroidism. Based on studies conducted by LLOYD, Inc., and in cooperation with two commercial laboratories, the following parameters for T-4 concentrations in canine serum have been established:
Normal (euthyroid) - 18 to 32 ng/mL (1.8 to 3.2 µg/dL)
Possible hypothyroid - 10 to 18 ng/mL (1.0 to 1.8 µg/dL)
Hypothyroid - less than 10 ng/mL (< 1.0 µg/dL). The animal should be showing some clinical signs associated with hypothyroidism.
A resting serum T-4 concentration of 18 ng/mL or above signifies that hypothyroidism is unlikely in dogs. Normally, the greater the T-4 concentration exceeds this value, the less likely that a dog is hypothyroid. A dog with a T-4 value below 18 ng/mL that is exhibiting signs of hypothyroidism should be considered for levothyroxine replacement therapy.
T-4 measurements should be made at 30 day intervals to establish the proper maintenance dose during a therapeutic trial with Thyro-Tabs Canine. A critical assessment of improvement in or resolution of clinical signs should be made after 12 weeks of levothyroxine sodium therapy. Further confirmation of the diagnosis could include withdrawal of the levothyroxine sodium therapy. A recurrence of clinical signs following cessation of therapy further supports the diagnosis. Correct diagnosis of hypothyroidism is important, since such a diagnosis normally commits an animal to life-long supplemental L-thyroxine replacement therapy. The principal objective of levothyroxine sodium administration is to achieve and maintain normal metabolism in the patient by providing an exogenous supply of synthetic L-thyroxine in amounts sufficient to maintain levels of the hormone within the animal’s normal physiologic range. Animal adaptation may necessitate regular monitoring of serum T-4 concentrations during the first several months of treatment to establish proper maintenance doses.
The TSH Response Test may be used to provide a definitive diagnosis in dogs with borderline resting serum T-4 values. The TSH dose, post-dose sampling times, and interpretation of pre- and post-TSH injection responses depend somewhat on the reference laboratory used.
Mode of actions: Levothyroxine sodium provided by Thyro-Tabs Canine cannot be distinguished from L-thyroxine endogenously secreted by the thyroid gland. L-thyroxine is a naturally circulating thyroid hormone released by the thyroid gland. The primary regulator of thyroid function is thyroid stimulating hormone (TSH), which is synthesized and secreted by the pars distalis of the adenohypophysis (anterior pituitary). The mediator from the hypothalamus which exerts a continuous influence over the pituitary release of TSH is thyrotropin-releasing hormone (TRH). Thyroid hormones influence virtually every body organ, either by their effect on growth and development or by the hormones’ metabolic effects.
Occurrence of canine hypothyroidism: Hypothyroidism usually occurs in middle-aged and older dogs although the condition will sometimes be seen in younger dogs of the larger breeds. Neutered animals of either sex are also frequently affected, regardless of age. The condition is usually primary, failure of the thyroid gland, because of lymphocytic thyroiditis or other loss of follicular epithelium and resulting atrophy of the gland. Secondary hypothyroidism is relatively rare and usually due to a destructive pituitary tumor.
Clinical signs of canine hypothyroidism: Not all dogs with hypothyroidism will have classical clinical signs and laboratory findings. The following list of clinical signs and laboratory findings may vary in dogs with hypothyroidism depending upon the degree and length of time of the thyroid dysfunction:
Nerve and muscle function: lethargy, lack of endurance, increased sleeping, reduced alertness and interest, impaired cerebral function and dulled mental attitude, hypotonus, stiff and slow movements, dragging of forelimbs, head tilt, disturbed balance.
Metabolism: decreased oxygen consumption and lower metabolic rate, sensitivity and intolerance to cold, low body temperature, cool skin, preference for warmth, increased body weight, constipation, poor exercise tolerance, slow heart rate, weak pulse, weak apex heart beat, and low voltage on ECG.
Reproduction: reproductive failure, abortion, stillbirth, live birth of weak young, delayed puberty, reduced libido, impaired spermatogenesis, irregular estrus and anestrus, galactorrhea.
Skin and hair: myxedema of face, blepharoptosis, atrophy of epidermis, thickening of dermis, surface and follicular hyperkeratosis, hyperpigmentation, coarse and sparse coat, dry, dull and brittle hair, slow regrowth and retarded turnover of hair, bilateral alopecia.
Laboratory findings: low serum T-4 concentrations, hypercholesterolemia, hypertriglyceridemia, elevated serum creatine kinase, anemia (normochromic, normocytic).
Contraindications
Levothyroxine sodium therapy is contraindicated in thyrotoxicosis, acute myocardial infarction, and uncorrected adrenal insufficiency. Other conditions in which the use of L-thyroxine replacement therapy may be contraindicated or should be instituted with caution include primary hypertension, euthyroidism, and pregnancy.
Precautions
The administration of levothyroxine sodium to dogs to be used for breeding purposes or in pregnant bitches has not been evaluated. There is evidence to suggest that administration to pregnant bitches may in some instances affect the normal development of the thyroid gland in the unborn pups.The clinical effects of levothyroxine sodium therapy are slow in being manifested. Overdosage of any thyroid drug may produce the signs and symptoms of thyrotoxicosis including, but not limited to: polydypsia, polyuria, polyphagia, reduced heat tolerance, and hyperactivity or personality change. Thyro-Tabs Canine 0.7 mg tablets contain FD&C Yellow #5 (tartrazine), which has been associated with allergic-type reactions (including bronchial asthma) in susceptible humans. It is unknown whether such a reaction could also occur in other animals.
Adverse Reactions
There are no specific adverse reactions associated with levothyroxine sodium administration at the recommended dosages. Overdosage will result in the signs of thyrotoxicosis listed above under precautions.Dosages: The initial recommended daily dose is 0.1 mg per 4.5 kg body weight in single or divided doses. Dosage is then adjusted by monitoring the T-4 blood levels of the dog every four weeks until an adequate maintenance dose is established. The usual daily maintenance dose is 0.1 mg per 4.5 kg. A maximum of 0.8 to 1.0 mg total daily dose will be sufficient in most dogs over 36 kg in body weight.
Administration: Thyro-Tabs Canine may be administered orally or placed in the food.
How Supplied
Thyro-Tabs Canine (levothyroxine sodium tablets), USP is available as scored, color-coded tablets in 8 concentrations: 0.1 mg - yellow; 0.2 mg - pink; 0.3 mg - green; 0.4 mg - maroon; 0.5 mg - white; 0.6 mg - purple; 0.7 mg - orange; 0.8 mg - blue; in bottles of 120.Storage
Store at controlled room temperature 15° - 30°C and protect from light and moisture.Keep out of reach of children.
References
1. Dickerson WM. Endocrine glands. In: Swenson MJ, Reece WO, eds. Dukes’ physiology of domestic animals. 11th ed. Ithaca, NY: Cornell University Press, 1993;629-664.
2. Chastain CB. Unusual manifestations of hypothyroidism in dogs. In: Kirk RW, Bonagura JD, eds. Current veterinary therapy Xl. Philadelphia: WB Saunders Co, 1992;330-334.
3. Peterson ME. The use of endocrine testing in the diagnosis and treatment of endocrine disorders, in Proceedings. 9th Annu Am Coll Vet Intern Med Forum 1991;109-114.
4. Panciera DL. Canine hypothyroidism. Part 1. Clinical findings and control of thyroid hormone secretion and metabolism. Compendium 1990;12:689-701.
5. McDonald LE. Hormones influencing metabolism. In: Booth NH, McDonald LE, eds. Veterinary pharmacology and therapeutics. 6th ed. Ames, IA: Iowa State University Press, 1998; 616-655.
6. Nelson RW, Ihle SL. Treating hypothyroidism through hormone supplementation. Vet Med 1987;82:153-156.
7. Nelson RW, Ihle SL. Hypothyroidism in dogs and cats: A difficult deficiency to diagnose. Vet Med 1987;82:60-70.
8. Chastain CB, Ganjam VK. The thyroid. In: Clinical endocrinology of companion animals. Philadelphia: Lea and Febiger,1986;110-175.
Manufactured by: LLOYD, Inc., Shenandoah, Iowa 51601, USA
Distributed by: Grey Wolf Animal Health Inc., Suite No. 85534, RPO Nortown, Toronto, ON M5N 0A2
Canada
1-844-400-GWAH (4924)
Rev. 0524
CPN: 1176023.3
Distributed by GREY WOLF ANIMAL HEALTH INC.
SUITE NO. 85534, RPO NORTOWN, TORONTO, ON, M5N 0A2
| Telephone: | 1-844-400-GWAH | |
| Website: | www.greywolfah.com | |
| Email: | support@greywolfah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
