Spryng for Equine
This treatment applies to the following species: Company: PetVivo
Company: PetVivo
Instructions for use
Veterinary Medical Device for Veterinary Use Only
Federal law restricts this device to use by or on the order of a licensed veterinarian.
Product Information
Spryng is a semi-transparent, colorless to pale yellow, insoluble hydrogel microparticulate for intra-articular injection in dogs, cats, and horses.
Each Spryng syringe contains > 90% collagen-elastin hydrogel microparticulate (CEHM) biomaterial dispersed in phosphate-buffered saline.
Sterile Spryng material is supplied in pre-filled luer-lock syringes available as 2 mL of product in a 5 mL syringe or a two-pack of 1 mL product in 3 mL syringe (needle not included). Each syringe is packaged aseptically in a sterilized peel pouch.
Spryng is biodegradable and produced in facilities with ISO 5 to ISO 7 environments.
Intended Use and Indications
Spryng is intended to aid in maintaining the mechanical homeostasis of tissues within and adjacent to the synovial lining of the joint.
Spryng is indicated to aid in the management of noninfectious sources of joint pain including, but not limited to, abnormal joint biomechanics, joint instability, osteoarthritis and congenital orthopedic conditions.
Contraindications
Spryng should not be used:
● If infection is suspected in the joint or surrounding tissues.
● In animals with immune-mediated conditions.
Warnings
Do not inject Spryng intravascularly. This can lead to blood vessel occlusion or emboli which may be fatal.
Not for use in humans.
Spryng has not been evaluated when combined with other intra-articular products in one syringe or co-administered during a single procedure.
Do not use if the package is open or the syringe is damaged.
Precautions
Use Spryng with caution in animals with a history of hypersensitivity.
Injections should be performed only by a licensed veterinarian skilled in the delivery of intra-articular (IA) injections.
Volume Recommendation
The volume of Spryng injected may vary from 0.25 mL up to 4 mL, depending on species, breed, intended joint, and joint capsule elasticity.
Need more guidance? Visit www.Sprynghealth.com > Veterinarian Resources or connect with one of our Technical Services Veterinarians.*
Directions For Use
Preparation
1. Animal restraint and sedation is recommended.
2. Shave or clip the hair if desired and perform a thorough surgical/aseptic prep over the joint to be injected.
3. Peel open the sealed pouch and dispense the contents into the sterile field. Alternatively, the opened pouch is suitable for use as a surgical field.
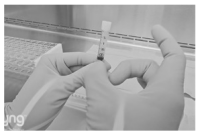
Administration
1. Vigorously flick and agitate the syringe to dislodge and disperse the particles homogeneously.
2. Insert a sterile needle (19-25g for horses or 22-25g for dogs and cats) into the joint space.
3. At the veterinarians discretion, inflammatory synovial fluid may be aspirated and discarded using a separate syringe while the needle remains in place.
4. Verify that the Spryng material is evenly dispersed within the syringe.
5. Attach the Spryng luer-lock syringe to the still-inserted needle.
6. Apply even pressure on the plunger while injecting.
7. Do not over-distend joints.
8. If additional product is needed, detach the empty syringe, then repeat the procedure with a new syringe maintaining aseptic technique.
9. When completed, remove the needle and syringe from the joint space and discard appropriately.
10. If a syringe is only partially used, discard the unused portion. Syringes are for single use only.
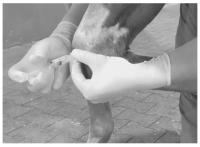
Equine Knee Injection
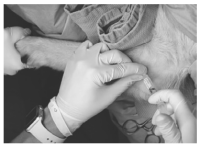
Canine Elbow Injection
Additional Administration Information
● Inflammation should be controlled prior to administration.
● Treatment with anti-inflammatory medications for at least five days pre-injection and ten days post-injection may improve medical outcomes and reduce the risk of adverse reactions.
● The optimal injection technique, location, and volume of Spryng administered may vary depending on the specific joint, patient, and the veterinarians clinical assessment.
● Multiple joints may be injected as a part of the same procedure.
● Improvement may be seen as early as 48 hours post-injection, but full benefits can take up to three weeks.
● Repeat or additional administration of Spryng may be considered based on the veterinarian’s assessment.
● Field experience suggests the benefits are long lasting and may extend beyond one year.
● A maximum annual administration frequency has not been established.
Risks and Side Effects
In field experience, less than 1% of animals experience adverse effects.
Animals may be uncomfortable within the first 48 hours of injection, similar to other intra-articular products.
Some animals may experience temporary swelling and discomfort in the injected joint beginning 7 to 14 days after injection, coinciding with the integration of the Spryng bioscaffold into the subsynovial tissue.
Veterinarians should use their clinical judgment to determine whether unexpected signs suggest a potentially septic joint.
If not septic in origin, these events normally resolve with prompt treatment with FDA-approved anti-inflammatory medications and rest.
Counseling for Owners
Discuss with owners the expected outcomes, including time to onset, duration, and level of improvement before administering Spryng.
Review contraindications, precautions, and potential complications. For optimal results, pre- and post-administration NSAIDs, strict stall/cage rest for 48 hours, and a gradual return to activity over two weeks should be followed.
Owners should be encouraged to contact their veterinarian immediately if unexpected signs occur.
Detailed guidance can be found in the Spryng Administration Guidelines and Best Practices on our Veterinarian Resources Page.
Potential Adverse Reactions
Adverse events should be promptly reported to PetVivo at 844-PET-VIVO (7388486) or info1@PetVivo.com
*Veterinarian Resources
For additional information, including FAQs, Administration Guidelines and Best Practices, Injection Volume Guidelines, safety data and case studies, visit: www.sprynghealth.com
Storage
Spryng should be stored at room temperature with a range of 40° - 90°F (5°-30°C). Use within the expiration date stated on the package.
Disposal
The syringe and any unused material must be discarded after a single treatment visit. Follow national, local, or institutional guidelines for use and disposal of medical sharp devices.
MADE IN THE USA
Manufactured by PetVivo, Inc. 5251 Edina Industrial Blvd, Minneapolis, MN 55439
844-PET-VIVO (738-8486)
www.petvivo.com
info1@petvivo.com
Spryng™ and OsteoCushion™ technology is a trademark of PetVivo Holdings Inc. This product is covered by U.S. Patent Nos. (US 8,153,591; US 9,107,937; US 9,999,705; US 10,850,006; US 11,890,371; US 11,975,121; CA 2,537,315; CA 2,583,561) and other pending applications and foreign patents. ©2024 PetVivo, Inc. All rights reserved.
TPD-19 Rev. 10-24
CPN: 2127000.3
5151 EDINA INDUSTRIAL BLVD., SUITE 575, MINNEAPLOLIS, MN, 55439
| Telephone: | 952-405-6216 | |
| Toll-Free: | 1-844-738-8486 | |
| Website: | www.petvivo.com | |
| www.petvivoanimalhealth.com | ||
| Email: | info1@petvivo.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
