Phenylzone Paste
This treatment applies to the following species: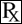 Company: Intervet/Merck Animal Health
Company: Intervet/Merck Animal Health
Product Information
(phenylbutazone)
VETERINARY - FOR HORSES ONLY
NADA #116-087, Approved by FDA.
Description
PHENYLZONE Paste is a synthetic, nonhormonal anti-inflammatory, antipyretic compound useful in the management of inflammatory conditions. The apparent analgesic effect is probably related mainly to the compound’s anti-inflammatory properties.
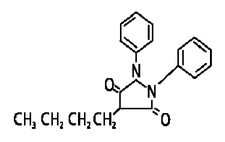
Chemically, phenylbutazone is 4-butyl-1,2-diphenyl-3,5-pyrazolidinedione. It is a pyrazolone derivative, entirely unrelated to the steroid hormones, and has the following structural formula:
Phenylzone Paste Indications
For the relief of inflammatory conditions associated with the musculoskeletal system in horses.
Contraindications
Use with caution in patients who have a history of drug allergy.
Warning
Do not use in horses intended for human consumption.
Precautions
Stop medication at the first sign of gastrointestinal upset, jaundice, or blood dyscrasia. Authenticated cases of agranulocytosis associated with the drug have occurred in man; fatal reactions, although rare, have been reported in dogs after long-term therapy. To guard against this possibility, conduct routine blood counts at weekly intervals during the early phase of therapy and at intervals of 2 weeks thereafter. Any significant fall in the total white count, relative decrease in granulocytes, or black or tarry stools, should be regarded as a signal for immediate cessation of therapy and institution of appropriate countermeasures. In the treatment of inflammatory conditions associated with infections, specific anti-infective therapy is required.
ADMINISTRATION AND DOSAGE:
Orally - 1 to 2 g of phenylbutazone per 500 lb (227 kg) of body weight, but not to exceed 4 g daily. Use a relatively high dose for the first 48 hours, then reduce gradually to a maintenance dose. Maintain lowest dose capable of producing desired clinical response.
Guidelines to Successful Therapy:
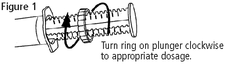
1. Important - To avoid overdosing and to ensure the correct first dose, move ring on plunger to the appropriate dosage position before administration (see Figure 1). The edge of the ring closest to the syringe barrel should be aligned to the gram/dosage mark on the plunger.
2. When administering PHENYLZONE Paste the oral cavity should be empty. Set ring on plunger to deliver the appropriate dosage based on weight (see Figure 1). Deposit paste on the back of tongue by depressing plunger.
3. Use a relatively high dose for the first 48 hours (not to exceed 4 g daily), then reduce gradually to a maintenance dose. Maintain lowest dose capable of producing desired clinical response.
4. Response to PHENYLZONE Paste therapy is prompt, usually occurring within 24 hours. If no significant clinical response is evident after 5 days, reevaluate diagnosis and therapeutic approach.
5. Many chronic conditions will respond to PHENYLZONE Paste therapy, but discontinuance of treatment may result in recurrence of symptoms.
Storage
Store at 15°-25° C (59°-77° F).
How Supplied
Syringes containing 12 g of phenylbutazone, NDC 0061-5040-01 (12 g).
KEEP OUT OF REACH OF CHILDREN
Phenylzone Paste Caution
Federal (U.S.A.) law restricts this drug to be used by or on the order of a licensed veterinarian.
Each syringe contains 12 g phenylbutazone
Each marking on the plunger contains:
|
Phenylbutazone |
1 g |
Manufactured for Intervet Inc d/b/a Merck Animal Health
Made in USA
Copyright© 2015 Intervet Inc, a subsidiary of Merck & Co., Inc. Madison, NJ 07940
All rights reserved.
12/15
|
Net wt. |
|
|
60 g |
156045 R4 / 140717 |
CPN: 1047147.4
Intervet Inc.
126 E. LINCOLN AVENUE, PO BOX 2000, Rahway, NJ, 07065
| Customer Service: | 800-521-5767 | |
| Technical Service (Companion Animal): | 800-224-5318 | |
| Technical Service (Livestock): | 800-211-3573 | |
| Website: | www.merck-animal-health-usa.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
