Imectro Chew
This treatment applies to the following species: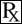 Company: Ceva Animal Health
Company: Ceva Animal Health
(ivermectin/pyrantel pamoate/praziquantel)
For oral use in dogs only.
Imectro Chew Caution
Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
IMECTRO™ Chew is a combination of three anthelmintics (ivermectin/pyrantel pamoate/praziquantel). The chews are available in four sizes in color-coded packages for oral administration to dogs according to their weight (see Dosage and Administration).
Imectro Chew Indications
For use in dogs to prevent canine heartworm disease by eliminating the tissue stage of heartworm larvae (Dirofilaria immitis) for a month (30 days) after infection and for the treatment and control of roundworms (Toxocara canis, Toxascaris leonina), hookworms (Ancylostoma caninum, Uncinaria stenocephala, Ancylostoma braziliense), and tapeworms (Dipylidium caninum, Taenia pisiformis).
Dosage and Administration
IMECTRO™ Chew should be administered orally at monthly intervals and the recommended minimum dose level of 6 mcg of ivermectin per kilogram (2.72 mcg/lb), 5 mg of pyrantel (as pamoate salt) per kg (2.27 mg/lb) and 5 mg of praziquantel per kg (2.27 mg/lb) of body weight, as follows:|
Dog Weight Pounds |
Chew Per Month |
Chew Size |
Ivermectin Content |
Pyrantel Pamoate Content |
Praziquantel Content |
|
6.0 to 12 |
1 |
Toy |
34 mcg |
28.5 mg |
28.5 mg |
|
12.1 to 25 |
1 |
Small |
68 mcg |
57 mg |
57 mg |
|
25.1 to 50 |
1 |
Medium |
136 mcg |
114 mg |
114 mg |
|
50.1 to 100 |
1 |
Large |
272 mcg |
228 mg |
228 mg |
IMECTRO™ Chew is recommended for dogs 8 weeks of age or older. For dogs over 100 lbs, use the appropriate combination of these soft chews.
Remove only one dose at a time from the packaging. Return the remaining chew(s) to their box to protect from light. The chew can be offered to the dog by hand or added, intact, to a small amount of dog food. Care should be taken to ensure that the dog consumes the complete dose. The treated dog should be observed for a few minutes after administration to confirm that none of the dose has been lost or rejected. If it is suspected that any of the dose has been lost, redosing is recommended.
IMECTRO™ Chew should be given at monthly intervals during the period of the year when mosquitoes (vectors), potentially carrying infective heartworm larvae, are active. The initial dose must be given within a month (30 days) after the dog’s first exposure to mosquitoes. The final dose must be given within a month (30 days) after the dog’s last exposure to mosquitoes.
When replacing another heartworm preventative product in a heartworm disease prevention program, the first dose of IMECTRO™ Chew must be given within a month (30 days) after the last dose of the former medication. A heartworm test should be performed prior to and 6 months after switching heartworm preventative products.
If the interval between doses exceeds a month (30 days), the effectiveness of ivermectin can be reduced. Therefore, for optimal performance, the chew must be given once a month on or about the same day of the month. If treatment is delayed, whether by a few days or many, immediate treatment with IMECTRO™ Chew and resumption of the recommended dosing regimen will minimize the opportunity for the development of adult heartworms.
Warnings
For use in dogs only. Keep this and all drugs out of reach of children and pets. In safety studies with ivermectin/pyrantel pamoate/praziquantel tablets, testicular hypoplasia was observed in some dogs receiving 3 and 5 times the maximum recommended dose monthly for 6 months (see Animal Safety).
In case of ingestion by humans, clients should be advised to contact a physician immediately. Physicians may contact a Poison Control Center for advice concerning cases of ingestion by humans.
Precautions
Use with caution in sick, debilitated, or underweight animals and dogs weighing less than 10 lbs (see Animal Safety). The safe use of this drug has not been evaluated in pregnant or lactating bitches.All dogs should be tested for existing heartworm infection before and 6 months after starting treatment with IMECTRO™ Chew, which is not effective against adult Dirofilaria immitis. Infected dogs should be treated to remove adult heartworms and microfilariae before initiating a heartworm prevention program.
While some microfilariae may be killed by the ivermectin in IMECTRO™ Chew at the recommended dose level, IMECTRO™ Chew is not effective for microfilariae clearance. A mild hypersensitivity-type reaction, presumably due to dead or dying microfilariae and particularly involving a transient diarrhea, has been observed in clinical trials with ivermectin alone after treatment of some dogs that have circulating microfilariae.
Adverse Reactions
In a field study with IMECTRO™ Chew, self-limiting adverse reactions, including vomiting, diarrhea, lethargy, difficulty swallowing, excessive salivation, increased water consumption, and coughing were reported. Self-limiting adverse reactions, including lethargy, limpness, salivation, shaking, diarrhea, decreased appetite, licking lips, and belching were reported between 20 minutes and 72 hours following treatment in a field study with ivermectin/pyrantel pamoate/praziquantel tablets.In field studies with ivermectin/pyrantel/praziquantel pamoate tablets, vomiting or diarrhea within 24 hours of dosing was rarely observed (1.1% of administered doses). The following adverse reactions have been reported in dogs following the use of ivermectin products: depression/lethargy, vomiting, anorexia, diarrhea, mydriasis, ataxia, staggering, convulsions, and hypersalivation.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Ceva Animal Health, LLC. at 1-800-999-0297. For additional information about adverse drug experience reporting for animal drugs, contact the FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
Effectiveness
Prevention of the tissue larval stage of heartworm (Dirofilaria immitis) and the elimination of the adult stage of hookworm (Ancylostoma caninum, Uncinaria stenocephala, Ancylostoma braziliense), roundworm (Toxocara canis, Toxascaris leonina), and tapeworm (Dipylidium caninum, Taenia pisiformis) infections in dogs was demonstrated in well-controlled laboratory studies.Palatability: In a field study of 132 dogs, IMECTRO™ Chew was offered once monthly for 3 months. The dogs voluntarily consumed 86.3% of the doses from the owner’s hand or from a bowl within 5 minutes, 13.0% accepted the dose when it was offered in food or administered by placing in the back of the dog’s tongue (pilling), and 0.7% of the doses were unable to be administered.
Animal Safety: Studies with ivermectin indicate that certain dogs of the Collie breed are more sensitive to the effects of ivermectin administered at elevated dose levels (more than 16 times the target dose level of 6 mcg/kg) than dogs of other breeds. At elevated doses, sensitive dogs showed more adverse reactions which included mydriasis, depression, ataxia, tremors, drooling, paresis, recumbency, excitability, stupor, coma and death. No signs of toxicity were seen at 10 times the recommended dose (27.2 mcg/lb) in sensitive Collies. Data from these studies support the safety of ivermectin products in dogs, including Collies, when used at the label recommended dose.
Because ivermectin and praziquantel are approximately 30% more bioavailable in the IMECTRO™ Chew than in the ivermectin/pyrantel pamoate/praziquantel tablets used in the following target animal safety studies, the margin of safety is narrower than reported in these studies. The potential for adverse reactions may be greater in individual dogs administered IMECTRO™ Chew than ivermectin/pyrantel pamoate/praziquantel tablets.
In a target animal safety study using ivermectin/pyrantel pamoate/praziquantel tablets, doses were administered to 8 week old Beagle puppies at one, three, and five times the maximum recommended dose of 12.5 mcg/kg ivermectin, 10.47 mg/kg pyrantel and 10.47 mg/kg praziquantel. The dogs were treated every 30 days for 6 months. Vomiting within 6 hours of dosing and soft or watery feces within 24 hours of dosing were observed. Other observations during the study were: ano-genital swelling, lethargy, head movements, shallow, audible or difficult breathing, and salivation. One dog in the 5X group had tremors and decreased activity. All of these signs were transient. No treatment was required. Histopathology showed testicular hypoplasia in the 3X and 5X groups (see Warnings).
In a laboratory safety study using ivermectin/pyrantel pamoate/praziquantel tablets, 12-week-old Beagle puppies receiving 3 and 5 times the recommended dose once weekly for 13 weeks demonstrated a dose-related decrease in testicular maturation compared to controls. In this study, all treated puppies had significantly higher cholesterol levels compared to untreated controls.
In a reproductive safety study, adult males were treated at 37.5 mcg/kg ivermectin, 31.4 mg/kg pyrantel and 31.4 mg/kg praziquantel every 14 days during two full spermatogenic cycles (112 days). The quality of semen and reproductive health were not affected by treatment. Treatment related vomiting and soft feces were reported during this study.
In a study of the effectiveness of ivermectin/pyrantel pamoate/praziquantel tablets for the treatment of Toxocara canis, one 8.1 lb, 72-day-old puppy died 6 days after administration of the label dose. This puppy and many other puppies in the study had high worm burdens and were reported to have diarrhea, sometimes bloody, frequently before and after treatment. Dehydration and signs of anemia (pale mucous membranes) were the only abnormal gross necropsy finding observed. No definitive cause was determined. In a 90-day field study using ivermectin/pyrantel pamoate/praziquantel tablets, the most serious adverse reactions (lethargy, limpness, and salivation) were seen in dogs weighing less than 10 lbs (see Precautions).
Storage Information: Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F to 86°F). Protect product from light.
How Supplied
IMECTRO™ Chew is available in four dosage strengths (see Dosage and Administration) for dogs of different weights. Each strength comes in a package of 6 chews.Approved by FDA under NADA # 141-441
Manufactured for:
Ceva Animal Health, LLC, Lenexa, KS 66215
©2021 Ceva Animal Health, LLC.
IMECTRO™ trademark is the property of Ceva Animal Health, LLC.
|
|
NET CONTENTS: |
|
|
6.0 - 12 LBS |
Contains 6 Chews: |
G39610B 03-G2-V1 500806 |
|
12.1 - 25 LBS |
Contains 6 Chews: |
G39620B 03-H2-V1 500807 |
|
25.1 - 50 LBS |
Contains 6 Chews: |
G39630B 03-I2-V1 500808 |
|
50.1 - 100 LBS |
Contains 6 Chews: |
G39640B 03-J2-V1 500809 |
CPN: 1328131.0
8735 ROSEHILL ROAD, STE. 300, LENEXA, KS, 66215
| Toll-Free: | 1-800-999-0297 | |
| Toll-Free Fax: | 877-777-5138 | |
| Website: | www.ceva.us |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
