GentaVed 100
This treatment applies to the following species: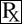 Company: Vedco
Company: Vedco
(Gentamicin Sulfate Solution)
100 mg/mL
Not for Use in Humans
For Use in Horses Only
GentaVed 100 Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Each mL of GentaVed® 100 (Gentamicin Sulfate Solution) contains Gentamicin sulfate veterinary equivalent to 100 mg gentamicin base; 2.4 mg sodium metabisulfite; 0.8 mg sodium sulfite, anhydrous; 0.1 mg edetate disodium; 10 mg benzyl alcohol as preservative; water for injection q.s.
CHEMISTRY: Gentamicin is a mixture of aminoglycoside antibiotics derived from the fermentation of Micromonospora purpurea. Gentamicin sulfate is a mixture of sulfate salts of the antibiotics produced in this fermentation. The salts are weakly acidic, freely soluble in water, and stable in solution.
ANTIBACTERIAL ACTIVITY: In vitro antibacterial activity has shown that gentamicin is active against most gram-negative and gram-positive bacteria isolated from domestic animals.1 Gentamicin is active against Pseudomonas aeruginosa, indole-positive and -negative Proteus species, Escherichia coli, Klebsiella species, Enterobacter species, Alcaligenes species, Staphylococcus species, and Streptococcus species.
Pharmacology
Studies in man indicate that recommended doses of gentamicin produce serum concentrations bactericidal for most bacteria sensitive to gentamicin within an hour after intramuscular injection; these concentrations last for 6 to 12 hours.2 Some 30% of the administered dose of gentamicin is bound by serum proteins and released as the drug is excreted.
Gentamicin is excreted almost entirely by glomerular filtration. High concentrations of the active form are found in the urine. Fifty to 100% of the gentamicin injected can be recovered unchanged within 24 hours from the urine of patients with normal renal function. A small amount is excreted into the bile.
TOXICITY STUDIES: No toxic effects were observed in rats given gentamicin sulfate 20 mg/kg/day for 24 days; in cats given 10 mg/kg/day for 40 days. Gentamicin sulfate given to dogs at 6 mg/lb/day, 6 days weekly for 3 weeks, caused no detectable kidney damage. At higher doses, impairment of equilibrium and renal function were observed in these species.
GentaVed 100 Indications
GentaVed® 100 (Gentamicin Sulfate Solution) is recommended for the control of bacterial infections of the uterus (metritis) in horses and as an aid in improving conception in mares with uterine infections caused by bacteria sensitive to gentamicin.
Bacteriologic studies should be conducted to identify the causative organism and to determine its sensitivity to gentamicin sulfate. Sensitivity discs of the drug are available for this purpose.
Dosage and Administration
The recommended dose is 20 to 25 mL (2.0 - 2.5 grams) gentamicin sulfate solution per day for 3 to 5 days during estrus. Each dose should be diluted with 200-500 mL of sterile physiological saline before aseptic uterine infusion.Contraindications
There are no known contraindications to this drug when used as directed.
PRECAUTION: If hypersensitivity to any of the components develops, or if overgrowth of nonsusceptible bacteria, fungi, or yeasts occurs, treatment with GentaVed® 100 (Gentamicin Sulfate Solution) should be discontinued and appropriate therapy instituted.
Although GentaVed® 100 (Gentamicin Sulfate Solution) is not spermicidal, treatment should not be given the day of breeding.
Warning
Do not use for horses intended for human consumption.Side Effects
There have been no reports of drug hypersensitivity or adverse side effects following the recommended intrauterine infusion of gentamicin sulfate solution combined with sterile physiological saline in mares.CONTACT INFORMATION: To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data sheet (SDS), contact Sparhawk Laboratories Inc at 1-800-255-6388 or 1-913-888-7500. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
How Supplied
GentaVed® 100 (Gentamicin Sulfate Solution), 100 mg per mL for intra-uterine use, is available in 100 mL and 250 mL multiple dose vials.Store between 2° and 30°C (36° and 86°F).
Protect from freezing.
For intra-uterine use in horses only.
Approved by FDA under ANADA # 200-395
Read accompanying directions carefully.
References
1. Hennessey, PW, et al. In vitro activity of gentamicin against bacteria isolated from domestic animals. Veterinary Medicine/Small Animal Clinician. Nov. 1971; 1118-1122.
2. Black, J, et al. Pharmacology of gentamicin, a new broad spectrum antibiotic. Antimicrob Agents and Chemother. 1963; 138-147.
Manufactured by
Sparhawk Laboratories, Inc., Lenexa, KS 66215, USA
Distributed By
VEDCO, INC., St. Joseph, MO 64507
|
NET CONTENTS: |
NDC |
|
|
|
100 mL Sterile Multiple Dose Vial |
50989-040-12 |
VINV-GENT-100M |
G-6336-04 Rev. 04-23 |
|
250 mL Sterile Multiple Dose Vial |
50989-040-15 |
VINV-GENT-250M |
G-6336-05 Rev. 04-23 |
CPN: 1094101.2
5503 CORPORATE DR., ST. JOSEPH, MO, 64507
| Telephone: | 816-238-8840 | |
| Toll-Free: | 888-708-3326 (888-70VEDCO) | |
| Fax: | 816-238-1837 | |
| Website: | www.vedco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
