EquiCoxib (firocoxib) Oral Solution for Horses
This treatment applies to the following species: Company: Aurora
Company: Aurora
9 mg/mL firocoxib
Non-steroidal anti-inflammatory drug for oral use in horses only.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
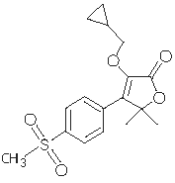
EquiCoxib™ (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs (NSAIDs). Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl) phenyl)-5,5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
EquiCoxib (firocoxib) Oral Solution for Horses Indications
EquiCoxib Oral Solution is administered for up to 14 days for the control of pain and inflammation associated with osteoarthritis in horses.
Dosage and Administration
Always provide the Client Information Sheet with the prescription. The recommended dosage of EquiCoxib (firocoxib) for oral administration in horses is 0.045 mg/lb (0.1 mg/kg) of body weight once daily for up to 14 days. In target animal safety studies, toxicity was seen at the recommended dose when the duration of treatment exceeded 30 days. Only administer EquiCoxib with the provided dosing syringe.
Each 1.25 mL volume will treat 250 pounds of body weight and each additional 0.25 mL volume corresponds to approximately a 50 lb weight increment. The provided dosing syringe is calibrated so that each line corresponds to a 50 lb weight increment. To deliver the correct dose, round the horse’s body weight up to the nearest 50 pound increment (if the body weight is an exact 50 pound increment, do not round up).
FOR ORAL USE ONLY. DO NOT INJECT EQUICOXIB. ONLY ADMINISTER WITH THE PROVIDED DOSING SYRINGE.
EquiCoxib Oral Dosing Guide
|
Body Weight (lb) |
Dose Volume (mL) |
|
250 |
1.25 mL |
|
500 |
2.5 mL |
|
750 |
3.75 mL |
|
1000 |
5 mL |
|
1250 |
6.25 mL |
1) Remove draw-off cap. Peel off the foil-backed seal from the bottle.
2) Screw the draw-off cap tightly back on the bottle.
3) Remove the seal from the top of the cap exposing the cross-hatched opening in the center of the silicone liner.
4) Remove the provided oral dosing syringe from its plastic cover.
5) Insert the oral dosing syringe firmly into the cross-hatched opening of the cap’s silicone liner.
6) Turn the bottle with attached syringe upside down. Pull back the syringe plunger until the widest portion of the plunger lines up with the line that corresponds with the animal’s weight. Each line between the 250 lb increments corresponds to 50 lb.
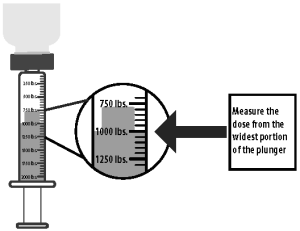
7) Turn the bottle with attached syringe right side up and separate the dosing syringe from the bottle.
8) Give orally according to your veterinarian’s instructions. DO NOT INJECT.
Contraindications
Horses with hypersensitivity to firocoxib should not receive EquiCoxib Oral Solution.
Warnings:
For oral use in horses only. Do not use in horses intended for human consumption.
Human Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Wash hands with soap and water after use. Consult a physician in case of accidental ingestion by humans.
Animal Safety: Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription.
Keep EquiCoxib in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Aurora Pharmaceutical at 1-888-215-1256 or www.aurorapharmaceutical.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Precautions
Horses should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data before and periodically during administration of any NSAID. Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription. See Information for Owner or Person Treating Horse section of the package insert.
Treatment with EquiCoxib should be terminated if signs such as inappetance, colic, abnormal feces, or lethargy are observed. As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Horses that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached or avoided. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforation, concomitant use of EquiCoxib Oral Solution with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. The concomitant use of protein bound drugs with EquiCoxib Oral Solution has not been studied in horses. The influence of concomitant drugs that may inhibit the metabolism of EquiCoxib Oral Solution has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. The safe use of EquiCoxib Oral Solution in horses less than one year in age, horses used for breeding, or in pregnant or lactating mares has not been evaluated. Consider appropriate washout times when switching from one NSAID to another NSAID or corticosteroid.
Adverse Reactions
In controlled field studies, 127 horses (ages 3 to 37 years) were evaluated for safety when given firocoxib at a dose of 0.045 mg/lb (0.1 mg/kg) orally once daily for up to 14 days. The following adverse reactions were observed. Horses may have experienced more than one of the observed adverse reactions during the study.
Adverse Reactions Seen In U.s. Field Studies
Firocoxib was safely used concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics, during the field studies. The safety data sheet (SDS) contains more detailed occupational safety information.
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Aurora Pharmaceutical Inc. at 1-888-215-1256 or www.aurorapharmaceutical.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at www.fda.gov/reportanimalae.
|
Adverse Reactions |
Firocoxib n=127 |
Active Control n=125 |
|
Abdominal pain |
0 |
1 |
|
Diarrhea |
2 |
0 |
|
Excitation |
1 |
0 |
|
Lethargy |
0 |
1 |
|
Loose stool |
1 |
0 |
|
Polydipsia |
0 |
1 |
|
Urticaria |
0 |
1 |
Information for Owner or Person Treating Horse: You should give a Client Information Sheet to the person treating the horse and advise them of the potential for adverse reactions and the clinical signs associated with NSAID intolerance. Adverse reactions may include erosions and ulcers of the gums, tongue, lips and face, weight loss, colic, diarrhea, or icterus. Serious adverse reactions associated with this drug class can occur without warning and, in some situations, result in death. Clients should be advised to discontinue NSAID therapy and contact their veterinarian immediately if any of these signs of intolerance are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care is initiated.
Clinical Pharmacokinetics / Pharmacodynamics:
Pharmacokinetics: When administered as a 0.045 mg/lb (0.1 mg/kg) dose in oral paste to adult horses with normal access to roughage, feed, and water, the absolute bioavailability of firocoxib from oral paste is approximately 79%. Following oral administration, drug peak concentration (Cmax) of 0.08 mcg/mL can be reached at 4 hours (Tmax) post-dosing. However, in some animals, up to 12 hours may be needed before significant plasma concentrations are observed. Little drug amount distributes into blood cells. The major metabolism mechanism of firocoxib in the horse is decyclopropylmethylation followed by glucuronidation of that metabolite. Based upon radiolabel studies, the majority of firocoxib is eliminated in the urine as the decyclopropylmethylated metabolite. Despite a high rate of plasma protein binding (98%), firocoxib exhibits a large volume of distribution (mean Vd(ss) = 1652 mL/kg). The terminal elimination half-life (T1/2) in plasma averages 30-40 hours after IV or oral paste dosing. Therefore, drug accumulation occurs with repeated dose administrations and steady state concentrations are achieved beyond 6-8 daily oral doses in the horse. Dose linearity exists from 1X-2X of 0.1 mg/kg/day.
Mode of action: EquiCoxib (firocoxib) is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic activity1 in animal models. Based on in vitro horse data, firocoxib is a selective inhibitor of prostaglandin biosynthesis through inhibition of inducible cyclooxygenase-2-isoenzyme (COX-2)2. Firocoxib selectivity for the constitutive isoenzyme, cyclooxygenase-1 (COX-1) is relatively low. However, the clinical significance of these in vitro selectivity findings has not been established.
Effectiveness
Two hundred fifty-three client-owned horses of various breeds, ranging in age from 2 to 37 years and weighing from 595 to 1638 lbs, were randomly administered firocoxib oral paste or an active control drug in multi-center field studies. Two hundred forty horses were evaluated for effectiveness and 252 horses were evaluated for safety. Horses were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall clinical improvement in a non-inferiority evaluation of firocoxib oral paste compared to an active control. At study’s end, 84.4% of horses treated with firocoxib oral paste were judged improved on veterinarians’ clinical assessment, and 73.8% were also rated improved by owners.
Horses treated with firocoxib oral paste showed improvement in veterinarian-assessed lameness, pain on manipulation, range of motion, and joint swelling that was comparable to the active control.
Animal Safety:
In a target animal safety study, firocoxib was administered orally to healthy adult horses (two male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 30 days. Administration of firocoxib at 0.3 and 0.5 mg/kg body weight was associated with an increased incidence of oral ulcers as compared to the control group but, no oral ulcers were noted with 0.1 mg/kg. There were no other drug-related adverse findings in this study.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (four males or male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 42 days. Administration of firocoxib at 0.1, 0.3 and 0.5 mg/kg body weight was associated with delayed healing of pre-existing oral (lip, tongue, gingival) ulcers. In addition, the incidence of oral ulcers was higher in all treated groups as compared to the control group.
Clinical chemistry and coagulation abnormalities were seen in several horses in the 0.5 mg/kg (5X) group. One 5X male horse developed a mildly elevated BUN and creatinine over the course of the study, prolonged buccal mucosal bleeding time (BMBT), and a dilated pelvis of the right kidney. Another 5X male had a similar mild increase in creatinine during the study but did not have any gross abnormal findings. One female in the 5X group had a prolonged BMBT, bilateral tubulointerstitial nephropathy and bilateral papillary necrosis. Tubulointerstitial nephropathy occurred in one 3X female, two 3X male horses, and the 5X female horse discussed above with the prolonged BMBT. Papillary necrosis was present in one 1X male horse and the 5X female horse discussed above. Despite the gross and microscopic renal lesions, all of the horses were clinically healthy and had normal hematology, clinical chemistry and urinalysis values.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (three females, two male castrates and one male per group) at 0, 0.25 mg/kg, 0.75 mg/kg and 1.25 mg/kg (2.5, 7.5 and 12.5X the recommended dose of 0.1 mg/kg) for 92 days. An additional group of three females, two male castrates and one male per group, was dosed at 1.25 mg/kg for 92 days but was monitored until Days 147-149. There were treatment-related adverse events in all treated groups. These consisted of ulcers of the lips, gingiva and tongue and erosions of the skin of the mandible and head. Gross and microscopic lesions of the kidneys consistent with tubulointerstitial nephropathy were seen in all treated groups. Papillary necrosis was seen in the 2.5X and 12.5X groups. In addition, several 12.5X horses had elevated liver enzymes (GGT, SDH, AST and ALT). One 2.5X horse had increased urine GGT and urine protein levels which was due to renal hemorrhage and nephropathy. Gastric ulcers of the margo plicatus and glandular area were more prevalent in the 2.5X and 7.5X groups, but not seen in the 12.5X group. The group of horses that were monitored until Days 147-149 showed partial to full recovery from oral and skin ulcers, but no recovery from tubulointerstitial nephropathy.
Storage Information:
Store below 77°F (25°C). Brief excursions up to 104°F (40°C) are permitted
How Supplied
EquiCoxib is available in 90 mL bottles, sufficient to treat a 1250 lb. horse for up to 14 days, and 400 mL bottles, sufficient to treat four 1250 lb. horses for up to 14 days.
References
1McCann ME, Rickes EL, Hora DF, Cunningham PK et al. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in cats with lipopolysaccharide-induced pyrexia. Am J Vet Res. 2005 Jul;66 (7):1278-84
2McCann ME, Anderson DR, Brideau C et al. In vitro activity and in vivo efficacy of a novel COX-2 inhibitor in the horse. Proceedings of the Academy of Veterinary Internal Medicine. 2002. Abstract 114, p.789.
Manufactured by: Aurora Pharmaceutical, Inc., Northfield, MN 55057
Approved by FDA under ANADA # 200-766
EquiCoxib™ is a trademark of Aurora Pharmaceutical, Inc.
IN 55-1756 04/2024
|
Net Contents: |
NDC |
Reorder No: |
|
|
90 mL |
51072-116-00 |
28008 |
IN 40-1755 10/2023 IN 50-1571 10/2023 |
|
400 mL |
51072-116-01 |
28009 |
IN 40-1796 06/2024 IN 50-1795 12/2023 |
CPN: 1544036.1
1196 HIGHWAY 3 SOUTH, NORTHFIELD, MN, 55057
| Telephone: | 888-215-1256 | |
| Website: | http://aurorapharmaceutical.com | |
| Email: | info@aurorapharmaceutical.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
