Encore
This treatment applies to the following species:(estradiol extended-release implants)
Each extended-release implant consists of 43.9 mg estradiol
Description
Each Encore (estradiol extended-release implant) silicone rubber implant contains 43.9 mg estradiol and is coated with sodium bicarbonate.
For subcutaneous ear implantation only.
INDICATIONS FOR USE:
● For increased rate of weight gain for up to 400 days in beef steer calves 2 months of age and older.
● For increased rate of weight gain for up to 400 days in growing beef steers on pasture (stocker, feeder, and slaughter).
● For increased rate of weight gain and improved feed efficiency for up to 400 days in growing beef steers and heifers fed in confinement for slaughter.
● This implant is not approved for repeated implantation (reimplantation) with this or any other cattle ear implant within each separate production phase:
• Beef steer calves 2 months of age and older
• Growing beef steers on pasture (stocker, feeder, and slaughter)
• Growing beef steers and heifers fed in confinement for slaughter
Safety and effectiveness following reimplantation have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been established.
Do not use in animals intended for subsequent breeding or in dairy cows.
Directions For Use
Insert one implant under the skin of the ear as directed in the detailed DIRECTIONS FOR IMPLANTATION below.
 |
WITHDRAWAL PERIODS AND RESIDUE WARNINGS No withdrawal period is required when used according to labeling. Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Implant pellets subcutaneously in ear only. Any other location is a violation of Federal law. Do not attempt salvage of implanted site for human or animal food. |
 |
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children.
ANIMAL SAFETY WARNINGS:
Increased sexual activity (bulling, riding and excitability) has been reported in animals implanted with Encore. Implanted animals should be observed for such signs particularly during the first few days after implanting and animals being excessively ridden (bullers) should be removed to prevent physical injury.Vaginal and rectal prolapse have been reported in heifers implanted with Encore.
Udder development, swollen or enlarged vulva and high tailheads may be observed in implanted heifers.
DIRECTIONS FOR IMPLANTATION:
A Compudose™ (estradiol extended-release implants) or Encore (estradiol extended-release implants) Implanter must be used to implant cattle.
COMPUDOSE/ENCORE IMPLANTER
Insert one implant under the skin of the ear.
1. Confine animal in a squeeze chute.
2. To reduce the possibility of infection and resulting implant loss, hygienic and antiseptic procedures should be followed during implantation.The ear should be clean and dry. The skin should be cleansed with a suitable antiseptic soap and dried prior to implanting. This is particularly important if the ears are contaminated with urine or feces.
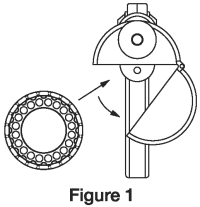
3. To load the Compudose/Encore Implanter remove the cartridge from the package, release the latch on the magazine, open and insert the cartridge. (Figure 1)
Close the magazine and latch. Advance the cartridge to the next implant by inserting the thumb into the magazine opening and turning the cartridge to the next stop.
Use a sharp needle. The needle should be cleaned and sterilized between each injection by wiping the exterior with a sponge, cloth, or gauze saturated with an appropriate disinfectant.
4. The implant should be deposited under the skin on the back side of the middle third of the ear. It should be placed between the skin and cartilage, avoiding major blood vessels.
Grasping the tip of the ear with one hand and the implanter in the other, penetrate the skin in the outer third of the ear. (see Figure 2)
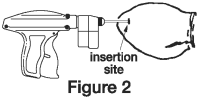
IMPORTANT: Do not penetrate cartilage.
5. Upon penetration, the needle should be fully inserted (Figure 3) between the skin and cartilage, avoiding major blood vessels. Full insertion of the needle is important to maximize implant retention.
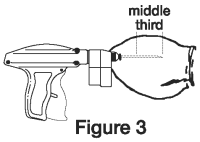
6. Pull the needle back as the implant is being deposited by squeezing the lever on the implanter grip. Figure 4 shows the implant in proper position in the middle third of the ear where the skin is tight.
7. Periodically remove the needle from the device and wash it in water. Before reusing, the needle should be disinfected and allowed to dry after shaking vigorously to remove excess water or disinfectant.
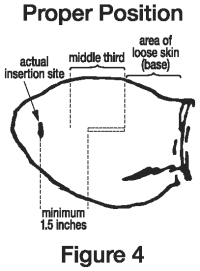
To maximize implant retention:
A. Fully insert the needle.
B. Deposit the implant in the middle third of the ear where the skin is tight.
C. Do not deposit the implant where the skin is loose in the third of the ear closest to the head.
The needle has been scientifically designed to maximize retention. When it becomes dull, use a new needle. If the needle is resharpened, sharpen only the point.
Failure to follow antiseptic implanting procedures, particularly when the ears are contaminated with fecal material, may result in infection and excessive implant loss. Implanting cattle during wet weather may increase infection and implant loss.
Carefully check the ears for implant loss approximately 4 weeks after implantation. If loss occurs, reimplant using recommended procedures.
How Supplied
Encore Implants are supplied in cartridges of 20 implants each.Storage Conditions
Store at 15 to 25°C (59-77°F). Do not refrigerate or freeze.
Approved by FDA under # NADA 118-123
Manufactured by a non-sterilizing process.
Elanco, Compudose, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2023 Elanco or its affiliates
Distributed by Elanco US, Inc., Greenfield, IN 46140, USA
Product of Germany
RESTRICTED DRUG (CALIFORNIA) - USE ONLY AS DIRECTED
To report side effects, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US, Inc. at 1-888-545-5973. For additional information about reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
May 2023
AH0952
PA104039X
W1a
CPN: 1131048.2
2500 INNOVATION WAY, GREENFIELD, IN, 46140
| Customer Service: | 317-276-1262 | |
| Technical Service: | 800-428-4441 | |
| Website: | www.elanco.us | |
| Email: | elanco@elanco.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
