Dormosedan Gel (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
detomidine hydrochloride oromucosal gel
DIN 02355957
Veterinary Use Only
Sedative
For Sublingual Use in Horses
Description
DORMOSEDAN® Gel (detomidine hydrochloride Ph.Eur.) is a synthetic alpha2-adrenoreceptor agonist with sedative properties. The chemical name is 1H imidazole, 4-[(2,3- dimethylphenyl) methyl]-hydrochloride. Detomidine hydrochloride is a white, crystalline, water-soluble substance having a molecular weight of 222.7.
The molecular formula is C12H14N2•HCl and the chemical structure is:
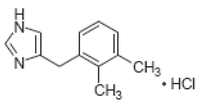
Each mL of DORMOSEDAN Gel contains 7.6 mg detomidine hydrochloride.
Dormosedan Gel Indications
DORMOSEDAN Gel is indicated for sedation and restraint in horses.
Dosage and Administration
DORMOSEDAN Gel produces sedation when administered sublingually at 0.040 mg/kg body weight (0.018 mg/lb body weight). DORMOSEDAN Gel must be placed beneath the tongue of the horse and is not meant to be swallowed. The dosing syringe delivers the product in 0.25 mL increments.
The following dosing table may be used to determine the correct dosage of DORMOSEDAN Gel (Table 1).
Table 1: Sublingual dosing of DORMOSEDAN Gel
|
Approximate body weight (kg) |
Range of doses (mg/kg) |
Approximate body weight (lb) |
Range of doses (mg/lb) |
Dose volume (mL) |
|
150-199 |
0.051 - 0.038 |
330 - 439 |
0.023 - 0.017 |
1.00 |
|
200-249 |
0.047 - 0.038 |
440 - 549 |
0.022 - 0.017 |
1.25 |
|
250-299 |
0.046 - 0.038 |
550 - 659 |
0.021 - 0.017 |
1.50 |
|
300-349 |
0.044 - 0.038 |
660 - 769 |
0.020 - 0.017 |
1.75 |
|
350-399 |
0.043 - 0.038 |
770 - 879 |
0.019 - 0.017 |
2.00 |
|
400-449 |
0.043 - 0.038 |
880 - 989 |
0.019 - 0.017 |
2.25 |
|
450-499 |
0.042 - 0.038 |
990 - 1099 |
0.019 - 0.017 |
2.50 |
|
500-549 |
0.042 - 0.038 |
1100 - 1209 |
0.019 - 0.017 |
2.75 |
|
550-600 |
0.041 - 0.038 |
1210 - 1320 |
0.019 - 0.017 |
3.00 |
Use impermeable gloves when handling this product. Remove the syringe from the outer carton. While holding the plunger, turn the ring-stop on the plunger until the ring is able to slide freely up and down the plunger. Position the ring in such a way that the side nearest the barrel is at the desired volume marking. Turn the ring to secure it in place. Make sure that the horse’s mouth contains no feed. Remove the cap from the tip of the syringe and save for cap replacement. Insert the syringe tip into the horse’s mouth from the side of the mouth, placing the syringe tip beneath the tongue at the level of the commissure of the mouth. Depress the plunger until the ring-stop contacts the barrel, depositing the product under the tongue.
The following picture demonstrates correct administration of DORMOSEDAN Gel beneath the tongue.
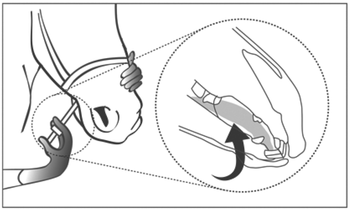
Take the syringe out of the horse’s mouth, recap the syringe and return it to the outer carton for disposal. Remove gloves for disposal.
For the best results, allow adequate time (a minimum of 40 minutes) between administration of DORMOSEDAN Gel and beginning the procedure. In general, horses show sedative effects lasting approximately 90-180 minutes.
Withhold food and water until the sedative effects of the product wear off.
Contraindications
DORMOSEDAN Gel is contraindicated in horses with known hypersensitivity to detomidine. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses as potentially fatal dysrhythmias may occur.
Do not use DORMOSEDAN Gel in horses with pre-existing atrio-ventricular (AV) or sino-atrial (SA) blocks, respiratory disease, or chronic renal failure.
CAUTIONS:
DORMOSEDAN Gel must be placed beneath the tongue of the horse. Unlike most oral veterinary products, this product is not meant to be swallowed. Swallowing could result in ineffectiveness. DORMOSEDAN Gel does not provide analgesia. Do not use for painful procedures.
Do not use with other sedative drugs because the effects may be additive.
Repeat dosing has not been evaluated.
The use of an alpha2-agonist reversal agent with DORMOSEDAN Gel has not been evaluated.
Before initiating any procedure, allow sedation to fully develop. Nervous or excited horses with high levels of endogenous catecholamines may exhibit a reduced pharmacological response to alpha2-adrenoceptor agonists like detomidine. In agitated horses, the onset of sedative effects could be slowed, or the depth and duration of effects could be diminished or nonexistent. When the product is administered, the animal should be allowed to rest in a quiet place for a minimum of 40 minutes.
Do not use DORMOSEDAN Gel in horses with cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation, or stress due to extreme heat, cold, fatigue, or high altitude. Protect treated horses from temperature extremes. As with all alpha2-adrenoceptor agonists, the potential for isolated cases of hypersensitivity exists, including paradoxical response (excitation).
DORMOSEDAN Gel has not been evaluated in ponies, miniature horses, or horses younger than one year of age.
DORMOSEDAN Gel has not been evaluated for use in breeding horses, and pregnant or lactating mares.
Warnings
Do not use in horses intended for food.
Keep out of reach of children.
Use impermeable gloves during drug administration and during procedures that require contact with the horse’s mouth. Following sublingual administration of detomidine hydrochloride oromucosal gel, drug concentrations up to 0.072 mg/mL were measured at 30 minutes post-treatment in equine saliva, equivalent to less than one percent of the original detomidine concentration in the gel. Mean drug concentrations fall to less than 0.010 mg/mL by 2 hours after drug administration, after which a slow decline occurs for several additional hours.
DORMOSEDAN Gel can be absorbed following direct exposure to skin, eyes, or mouth, and may cause irritation. Skin and mucosal contact with the product should be avoided.
In case of accidental eye exposure, rinse abundantly with fresh water. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing.
Appropriate precautions should be taken while handling and using gel syringes. Accidental exposure could cause adverse reactions, including sedation, hypotension, and bradycardia. Seek medical attention immediately but do not drive because sedation or changes in blood pressure may occur.
Individuals with cardiovascular disease (for example, hypertension or ischemic heart disease) should take special precautions to avoid exposure to this product.
Caution should be exercised when handling sedated animals. Handling or any other sudden stimuli, including noise, may cause a defense reaction in an animal that appears to be heavily sedated.
Rare cases of human abuse of detomidine products have been reported. DORMOSEDAN Gel should be managed to prevent the risk of diversion, through such measures as restriction of access and the use of drug accountability procedures appropriate to the clinical setting.
The material safety data sheet (MSDS) contains more detailed occupational safety information. To report adverse reactions in users or to obtain a copy of the MSDS for this product call 1-800-461-0917.
Note to physician: This product contains an alpha2-adrenoreceptor agonist.
Adverse Reactions
In a U.S. field study of 270 horses sedated to facilitate completion of various veterinary and husbandry procedures, the following adverse reactions were reported in 202 horses treated with DORMOSEDAN Gel and 68 horses treated with placebo:
Table 2: Adverse reactions (number of horses) during the clinical field study
|
Clinical Sign |
DORMOSEDAN Gel |
Placebo |
|
Sweating |
20 |
0 |
|
Penile relaxation |
12 |
0 |
|
Bradycardia (≤20 bpm) |
11 |
0 |
|
Second degree AV block |
9 |
0 |
|
Frequent urination |
9 |
0 |
|
Piloerection |
4 |
0 |
|
Marked ataxia |
3 |
0 |
|
Facial/oral edema |
3 |
0 |
|
Hypersalivation |
2 |
0 |
|
Nasal discharge |
2 |
0 |
|
Flatulence |
1 |
0 |
|
Muscle tremors |
1 |
1 |
|
Epiphora |
1 |
0 |
|
Pale mucous membranes |
1 |
0 |
|
Swollen sheath |
1 |
0 |
In a laboratory study, transient erythema of the mucous membranes was seen in 2 (of 8) horses that received the recommended dose of DORMOSEDAN Gel.
Mild ataxia (horse stable but swaying slightly) was observed in 54% of DORMOSEDAN Gel-treated horses and in 4% of the placebo-treated horses at 40 minutes post-treatment. Moderate ataxia was observed in 25% of DORMOSEDAN Gel-treated horses (0% placebo) at 40 minutes post-treatment. Moderate to marked ataxia continued to 90 minutes for 5% and to 120 minutes for 4% of DORMOSEDAN Gel-treated horses.
Clinical Pharmacology
Detomidine is a potent non-narcotic alpha2-adrenoceptor agonist which produces sedation with a central effect inhibiting the transmission of noradrenalin-mediated nervous impulses. Blood pressure is initially increased due to peripheral vasoconstriction, subsequently dropping to normal or slightly below normal levels. Vasoconstriction may cause mucous membranes to appear pale or mildly cyanotic. This initial vasopressor response is accompanied by a compensatory marked decrease in heart rate mediated by a vagal baroreceptor. The peripheral pulse may feel weak, and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by first and second degree atrioventricular blocks. Other arrhythmias may occur. Detomidine also decreases the respiratory rate and decreases body temperature. Detomidine causes depression of gastrointestinal motility due to decrease in smooth muscle activity, increases blood glucose levels due to inhibition of insulin release, and increases production of urine 2 to 4 hours after treatment. In some horses, sweating, salivation and slight muscle tremors may be seen. Partial, transient penis prolapse may occur in stallions and geldings. Because of continued lowering of the head during sedation, mucus discharges from the nose with occasional swelling of the head, particularly around the eyes, may be seen.
Detomidine is oxidized mainly in the liver. Most metabolites are excreted in the urine. Halflife (T1/2) is 1-2 hours. Detomidine is rapidly distributed; volume of distribution (Vd) varies between 0.69 L/kg and 1.89 L/kg. Protein binding is about 85%.
Detomidine is a high extraction ratio drug. Alterations in liver blood flow (the site of detomidine metabolism) can change the rate of drug clearance and, consequently, drug exposure. The sedative effects of detomidine (using head droop as a marker for sedation) are highly correlated to blood concentration, regardless of the route of administration.
First pass effect results in a very small portion of the drug reaching the systemic circulation if it is swallowed. Sedation achieved with DORMOSEDAN Gel is attributable to sublingual drug absorption. Peak concentrations occur approximately 1.83 hours after sublingual administration of DORMOSEDAN Gel. The peak concentrations observed after administration of the oromucosal gel are approximately 40% of those observed after intramuscular injection of detomidine hydrochloride injectable sterile solution. The absolute bioavailability of detomidine in the oromucosal gel is 22%.
Effectiveness
A prospective, randomized, masked multi-center study was conducted to evaluate under field conditions, whether DORMOSEDAN Gel provided sufficient sedation and restraint in horses to successfully conduct procedures requiring administration of a sedative. Two hundred and seventy client-owned horses of any breed or sex were sedated to facilitate grooming (including cleaning of the prepuce), hoof care, floating teeth (manually), passage of a nasogastric tube or endoscope, or radiography. Horses were enrolled in the study if they were a yearling or older, in satisfactory body condition, and had a history of requiring sedation or other means of strong restraint to enable similar procedures to be carried out. Horses were randomly assigned to receive DORMOSEDAN Gel sublingually at 0.040 mg/kg or placebo gel. After administration of treatment, each horse’s level of sedation, degree of ataxia, heart rate and rhythm, and respiratory rate were assessed and measured to recovery. After an appropriate period of time elapsed to allow sedation to develop, a study veterinarian assessed and scored the ability to attempt and to complete the veterinary or husbandry procedure.
One hundred and twenty nine DORMOSEDAN Gel-treated and 42 placebo-treated horses were included in the statistical analysis of effectiveness. Ninety nine horses were excluded from the analysis due to failure to meet inclusion criteria or due to major protocol deviations. The veterinary or husbandry procedure was successfully completed for 98 of 129 DORMOSEDAN Gel-treated horses (76%), but only 3 of 42 placebo-treated horses (7%) (Table 3). The difference between the two treatments was statistically significant (p=0.0005).
Table 3: Treatment success rates (number of horses) by treatment group
|
Ability to perform the procedure score* |
DORMOSEDAN Gel |
Placebo |
|
0 |
16 |
38 |
|
1 |
15 |
1 |
|
2 |
44 |
2 |
|
3 |
54 |
1 |
|
Success (score 2 or 3) |
98 |
3 |
* 0: Poor - Strong resistance. 1: Fair - Moderate resistance. 2: Good - Some resistance, but the procedure could be performed. 3: Excellent - Procedure could be easily performed with insignificant resistance.
The following success rates with DORMOSEDAN Gel were recorded for electric clipping of hair (48%), cleaning the prepuce (81%), manual floating of teeth (89%), hoof trimming or shoeing (86%), passage of a nasogastric tube or endoscope (80%), or radiography (74%). At 40 minutes post-treatment, 94% of DORMOSEDAN Gel-treated horses showed minimal, moderate or marked sedation compared with 14% of the horses treated with placebo. All DORMOSEDAN Gel-treated horses had recovered from sedation by 240 minutes post-treatment.
DORMOSEDAN Gel was easy to administer. The product was correctly administered sublingually (under the tongue) in 97% of horses with only mild or no objection.
ANIMAL SAFETY:
In a multiple dose target animal safety study, DORMOSEDAN Gel was administered on three consecutive days to 6 horses per treatment group at 0, 1, 3 and 5 times the recommended label dose of 0.040 mg/kg.
The recommended dose (1X) induced sedation. Head droop caused transient edema of the head area, nasal/ocular discharge, and congestion of oral mucous membranes. Ataxia, sweating, and reversible penile prolapse were observed. Erythematous mucous membranes were seen at the area of dose application in 2/6 horses. Transient reductions were seen in heart rate, respiratory rate, and gut motility. Electrocardiography revealed increased incidences of vagally mediated arrhythmias (sinus arrhythmia, sinus block, 1st and 2nd degree atrioventricular block) as well as atrial or ventricular premature beats in the majority of horses. No clinical abnormalities were associated with the transient arrhythmias. Excessive or erratic urination were seen in isolated cases.
Similar treatment related findings were seen in horses receiving 3X and 5X doses. In most cases the incidence, severity, and duration of the findings were dose dependent. All findings in all dose groups were representative of the alpha2-adrenoreceptor drugs used in horses.
Storage
Store between 15 and 30°C. Protect from light.PRESENTATION: DORMOSEDAN Gel is supplied in 3.0 mL graduated oral dosing syringe.
®Dormosedan is a registered trademark of Orion Corporation; Zoetis Canada Inc., Licensee.
© Zoetis Canada Inc., 2014
Developed and manufactured by: ORION PHARMA, Orion Corporation, Turku, Finland
Distributed by: Zoetis Canada Inc., Kirkland QC H9H 4M7
6291-11-1
136726-2
CPN: 1198445.2
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
