Dexium Injection For Bovine & Equine
This treatment applies to the following species: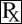 Company: Bimeda
Company: Bimeda
(dexamethasone) SOLUTION
ANADA 200-312, Approved by FDA
2 mg/mL
Veterinary
Sterile
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Dexium Injection For Bovine & Equine Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Dexamethasone Solution is a synthetic analogue of prednisolone, having similar but more potent anti-inflammatory therapeutic action and diversified hormonal and metabolic effects. Modification of the basic corticoid structure as achieved in Dexium® offers enhanced anti-inflammatory effect compared to older corticosteroids. The dosage of Dexium® required is markedly lower than that of prednisone and prednisolone.
Dexium® is not species-specific; however, the veterinarian should read the sections on INDICATIONS, DOSAGE, SIDE EFFECTS, CONTRAINDICATIONS, PRECAUTIONS, and WARNINGS before this drug is used.
Dexium® is intended for intravenous or intramuscular administration. Each mL contains 2 mg dexamethasone, 500 mg polyethylene glycol 400, 9 mg benzyl alcohol, 1.8 mg methylparaben and 0.2 mg propylparaben as preservatives, 4.75% alcohol, HCl to adjust pH to approximately 4.9, water for injection q.s.
EXPERIMENTAL STUDIES: Experimental animal studies on dexamethasone have revealed it possesses greater anti-inflammatory activity than many steroids. Veterinary clinical evidence indicates dexamethasone has approximately 20 times the anti-inflammatory activity of prednisolone and 70 to 80 times that of hydrocortisone. Thymus involution studies show dexamethasone possesses 25 times the activity of prednisolone. In reference to mineralocorticoid activity, dexamethasone does not cause significant sodium or water retention. Metabolic balance studies show that animals on controlled and limited protein intake will exhibit nitrogen losses on exceedingly high dosages.
Dexium Injection For Bovine & Equine Indications
Dexium® is indicated for the treatment of primary bovine ketosis and as an anti-inflammatory agent in the bovine and equine.
As supportive therapy, Dexium® may be used in the management of various rheumatic, allergic, dermatologic, and other diseases known to be responsive to anti-inflammatory corticosteroids. Dexium® may be used intravenously as supportive therapy when an immediate hormonal response is required.
Bovine Ketosis
Dexium® is offered for the treatment of primary ketosis. The gluconeogenic effects of Dexium®, when administered intramuscularly, are generally noted within the first 6 to 12 hours. When Dexium® is used intravenously, the effects may be noted sooner. Blood sugar levels rise to normal levels rapidly and generally rise to above normal levels within 12 to 24 hours. Acetone bodies are reduced to normal concentrations usually within 24 hours. The physical attitude of animals treated with Dexium® brightens and appetite improves, usually within 12 hours. Milk production, which is suppressed as a compensatory reaction in this condition, begins to increase. In some instances, it may even surpass previous peaks. The recovery process usually takes from 3 to 7 days.
Supportive Therapy
Dexium® may be used as supportive therapy in mastitis, metritis, traumatic gastritis, and pyelonephritis, while appropriate primary therapy is administered. In these cases, the corticosteroid combats accompanying stress and enhances the feeling of general well-being.
Dexium® may also be used as supportive therapy in inflammatory conditions such as arthritic conditions, snake bite, acute mastitis, shipping fever, pneumonia, laminitis, and retained placenta.
Equine
Dexium® is indicated for the treatment of acute musculoskeletal inflammations, such as bursitis, carpitis, osselets, tendonitis, myositis, and sprains. If boney changes exist in any of these conditions, joints, or accessory structures, a response to Dexium® cannot be expected. In addition, Dexium® may be used as supportive therapy in fatigue, heat exhaustion, influenza, laminitis, and retained placenta provided that the primary cause is determined and corrected.
ADMINISTRATION AND DOSAGE: Therapy with Dexium®, as with any other potent corticosteroid, should be individualized according to the severity of the condition being treated, anticipated duration of steroid therapy, and animal’s threshold or tolerance for steroid excess.
Treatment may be changed over to Dexium® from any other glucocorticoid with proper reduction or adjustment of dosage.
Bovine: Dexium®: 5 - 20 mg intravenously or intramuscularly.
Equine: Dexium®: 2.5 - 5 mg intravenously or intramuscularly.
Contraindications
Except for emergency therapy, do not use in animals with chronic nephritis and hyper-corticalism (Cushing’s syndrome). Existence of congestive heart failure, diabetes, and osteoporosis are relative contraindications. Do not use in viral infections during the viremic stage.
Precautions
Animals receiving Dexium® should be under close observation. Because of the anti-inflammatory action of corticosteroids, signs of infection may be masked and it may be necessary to stop treatment until a further diagnosis is made. Overdosage of some glucocorticoids may result in sodium retention, fluid retention, potassium loss, and weight gain.Dexium® may be administered to animals with acute or chronic bacterial infections providing the infections are controlled with appropriate antibiotic or chemotherapeutic agents.
Doses greater than those recommended in horses may produce transient drowsiness or lethargy in some horses. The lethargy usually abates in 24 hours.
Use of corticosteroids, depending on the dose, duration, and specified steroid, may result in inhibition of endogenous steroid production following drug withdrawal. In patients presently receiving or recently withdrawn from systemic corticosteroid treatments, therapy with a rapidly acting corticosteroid should be considered in unusually stressful situations.
Warnings
Clinical and experimental data have demonstrated that corticosteroids administered orally or parenterally to animals may induce the first stage of parturition when administered during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have produced cleft palate. Other congenital anomalies including deformed forelegs, phocomelia, and anasarca have been reported in offspring of dogs which received corticosteroids during pregnancy.
A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
Side Effects
Side effects, such as SAP and SGPT enzyme elevations, weight loss, anorexia, polydipsia, and polyuria have occurred following the use of synthetic corticosteroids in dogs. Vomiting and diarrhea (occasionally bloody) have been observed in cats and dogs. Cushing’s syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.Corticosteroids reportedly cause laminitis in horses.
How Supplied
Dexium®, 2 mg per mL, 100 mL multiple dose vial.STORE BETWEEN 2°C - 30°C (36°F - 86°F).
PROTECT FROM FREEZING.
® Dexium is a registered trademark of Bimeda, Inc.
Manufactured by: Bimeda-MTC Animal Health Inc., Cambridge, ON Canada
www.bimedamtc.com
Manufactured for: Bimeda, Inc., Le Sueur, MN 56058
www.bimeda.com
|
Net Contents: |
Product No. |
|
|
100 mL |
1DEX022 |
8DEX018H REV. 04/13 |
CPN: 1399071.3
Div. Cross Vetpharm Group, Ltd.
475 NORTH MARTINGALE ROAD, SUITE 1200, SCHAUMBURG, IL, 60173
| Telephone: | 630-928-0361 | |
| Fax: | 630-928-0362 | |
| Website: | www.bimedaus.com | |
| Email: | us-sales@bimeda.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
