Compudose Implants (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
Estradiol
Controlled Release Implant
DIN 00590932
Active Ingredient
Each implant contains 25.7 mg estradiol impregnated in silicone rubber coated with not less than 0.5 mg oxytetracycline as a local antibacterial.
For subcutaneous ear implantation in beef cattle only.
Compudose Implants Indications
1. For increased rate of weight gain in suckling and growing steers greater than 80 kg (175 lb) body weight.
2. For increased rate of weight gain and improved feed efficiency in feedlot steers and heifers greater than 260 kg (575 lb) body weight.
For maximum response while on pasture, excellent nutrition must be provided by the pasture alone or with grain supplementation.
USE: Each Compudose controlled release implant will provide an effective daily dose of estradiol for at least 200 days.
Directions For Use
Insert one implant under the skin of the ear as directed in the leaflet.
Warnings
1. Do not use in calves to be processed for veal.
2. Implant Compudose in the ear only; any other location may result in condemnation of the carcass. Do not attempt to salvage the implanted ear for human or animal food.
3. KEEP OUT OF REACH OF CHILDREN.
EQUIPMENT: A Compudose Implanter must be used. Implanters and replacement needles are available from your Compudose supplier.
DIRECTIONS FOR IMPLANTATION:
Insert one implant under the skin of the ear.
1. Immobilize the animal in a squeeze chute or by other means.
2. To reduce the possibility of infection and resulting implant loss, hygienic and antiseptic procedures should be followed during implantation. The ear should be clean and dry. The skin should be cleansed with a suitable antiseptic soap and dried prior to implanting. This is particularly important if the ears are wet or contaminated with urine or feces.
3. To load the Compudose Implanter, remove the cartridge from the package, release the latch on the magazine, open and insert the cartridge. (Figure 1)
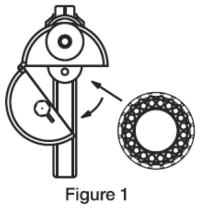
Close the magazine and latch. Advance the cartridge to the next implant by inserting the thumb into the magazine opening and turning the cartridge to the next stop.
Use a sharp needle. The needle should be cleaned and sterilized between each injection by wiping the exterior with a sponge, cloth, or gauze saturated with an appropriate disinfectant. The presence of excessive moisture inside the needle may result in dissolving the oxytetracycline (OTC) from the implant surface and contribute to accumulation of OTC inside the needle.
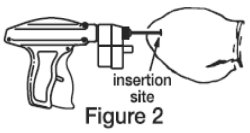
4. The implant should be deposited under the skin on the back side of the middle third of the ear. It should be placed between the skin and cartilage, avoiding major blood vessels. Grasping the tip of the ear with one hand and the implanter in the other, penetrate the skin in the outer third of the ear. (Figure 2)
IMPORTANT: DO NOT PENETRATE CARTILAGE.
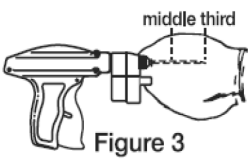
5. Upon penetration, the needle should be fully inserted (Figure 3) between the skin and cartilage, avoiding major blood vessels. Full insertion of the needle is important to maximize implant retention.
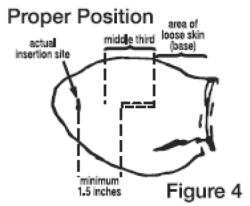
6. Pull the needle back as the implant is being deposited by squeezing the lever on the implanter grip. Figure 4 shows the implant in proper position in the middle third of the ear where the skin is tight.
7. After repeated use, sufficient oxytetracycline (OTC) from the implant may accumulate inside the needle to impede implant passage. Periodic removal of the needle from the device and washing it in water will prevent such accumulation. Before reusing, the needle should be disinfected and allowed to dry after shaking vigorously to remove excess water or disinfectant.
CONTRAINDICATION: Animals administered an implant at 260 kg (575 lb) body weight need not be reimplanted.
Precautions
To maximize implant retention:A. Fully insert the needle.
B. Deposit the implant in the middle third of the ear where the skin is tight.
C. Do not deposit the implant where the skin is loose in the third of the ear closest to the head.
The needle has been scientifically designed to maximize retention. When it becomes dull, use a new needle. If the needle is resharpened, sharpen only the point.
CAUTIONS:
1. Increased sexual activity (bulling, riding and excitability) has been reported in animals implanted with estradiol implants. Implanted animals should be observed for such signs particularly during the first few days after implanting and animals being excessively ridden (bullers) should be removed to prevent physical injury.
2. Carefully check the ears for implant loss approximately 4 weeks after implantation. If loss occurs, reimplant using recommended procedures.
3. Failure to follow antiseptic implanting procedures, particularly when the ears are wet or contaminated with fecal material, may result in infection and excessive implant loss. Dipping animals in contaminated solutions immediately following implanting may also result in infection and loss of implants.
4. Vaginal and rectal prolapse have been reported in heifers implanted with anabolic estrogens.
5. Do not use in animals intended for breeding purposes.
Side Effects
High tailheads, udder development and swollen vulvas may occur in cattle treated with anabolic estrogens.Storage
Store between 15 to 25°C. Do not refrigerate or freeze.How Supplied
Compudose Implants are supplied in boxes of 100 implants packaged as 5 cartridges of 20 implants each.MANUFACTURER’S NAME AND ADDRESS: Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
DATE: April 2022
Compudose, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
|
Net: |
|
|
|
5 x 20 Implants |
AH0327 |
17May2022 |
CPN: 1231148.2
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
