Cepesedan (Canada)
This treatment applies to the following species: Company: Modern Veterinary Therapeutics
Company: Modern Veterinary Therapeutics
(Detomidine hydrochloride injection - 10 mg/mL)
DIN 02335441
Injectable sedative and analgesic for use in horses only - Sterile Solution.
For veterinary use only.
Active Ingredient - Each mL contains:
|
Detomidine hydrochloride |
10 mg |
Non-medicinal ingredients - Each mL contains:
|
Methylparahydroxybenzoate (preservative) |
1 mg |
|
Sodium chloride |
5.9 mg |
|
Water for injection |
q.s |
Description
CEPESEDAN™ is a synthetic alpha-2 adrenoceptor agonist with sedative and analgesic properties. The chemical name is 1H-imidazole, 4-[(2,3-dimethylphenyl)methyl]-hydrochloride and the active ingredient’s name is detomidine hydrochloride. It is a white, crystalline, water soluble substance having a molecular weight of 222.7. The molecular formula is C12H14N2•HCl.
Chemical Structure:
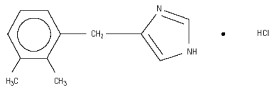
Cepesedan Indications
CEPESEDAN™ is indicated for use as a sedative and analgesic to facilitate minor surgical and diagnostic procedures in mature horses and yearlings.
Dosage and Administration
Administer CEPESEDAN™ intravenously or intramuscularly at the rates of 20 or 40 µg detomidine hydrochloride per kg of body weight (0.2 to 0.4 mL of CEPESEDAN™ per 100 kg or 220 lbs.), depending upon the depth and duration of sedation and analgesia required. Analgesia is more pronounced when the drug is given intravenously. Sedative and analgesic effects will usually occur in 2 to 5 minutes and will persist for 30 minutes to 2 hours, depending upon the dosage and route of administration.Prior to and following injection, the animal should be allowed to rest quietly. The full effect should be reached within 2 to 4 minutes after I.V. injection and 3 to 5 minutes after intramuscular injection.
Contra-indications: Not to be used in horses with pre-existing AV or SA block, with severe coronary insufficiency, cerebrovascular disease, respiratory disease, or chronic renal failure. Detomidine hydrochloride should not be used in breeding horses since the potential risk has not been assessed in either stallions or mares.
Cepesedan Caution
The drug is a potent α-2 agonist which should be used with extreme caution with other sedatives, analgesics, or anesthetics as these are likely to produce additive effects. The use of epinephrine should be avoided, except in anesthetic emergency, since epinephrine may potentiate the effects of α-2 agonists. Careful consideration should be given prior to the administration of CEPESEDAN™ to horses in endotoxic or traumatic shock or approaching shock, to horses with advanced liver or kidney disease or other preexisting conditions of stress such as extreme heat or cold, high altitude, or fatigue. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses as potentially fatal dysrhythmias may occur. Protect treated horses from temperature extremes. Food and water should be withheld until the sedative effect of CEPESEDAN™ has worn off.
Warning
This drug is not to be administered to horses that are to be slaughtered for use in food. Keep out of reach of children.Adverse Reactions
As with all α2-agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation) exists. There has been an occasional appearance of arrhythmia, hypertension, and bradycardia in horses treated with detomidine hydrochloride. Piloerection, sweating and salivation, and, occasionally, slight muscle tremors are frequently seen after administration. Partial transient penis prolapse may be seen. Decreased heart rate and respiratory rate may occur. Incoordination or staggering is usually seen only during the first 3 to 5 minutes after injection, until animals have secured a firm footing. Because of continued lowering of the head during sedation, passive congestion may occur occasionally in the lips, the facial area, and the upper airways. Tying the head in a slightly elevated position generally prevents these effects.NOTE TO USERS: Animals should not be left unattended during the development and duration of sedation. Allowing the horse to stand quietly for 5 minutes prior to and after injection may improve the response to CEPESEDAN™. Some horses, although apparently deeply sedated, may still respond to external stimuli. Routine safety measures should be employed to protect practitioners and handlers.
Overdosage: Detomidine hydrochloride is tolerated in horses at up to 200 µg per kg of body weight (10 times the low dosage and five times the high dosage). In safety studies in horses, detomidine hydrochloride at 400 µg per kg body weight, administered once a day for 3 consecutive days produced microscopic foci of myocardial necrosis in 1 of 8 horses.
Clinical Pharmacology
CEPESEDAN™, a non-narcotic sedative and analgesic, is a potent α-2 adrenoceptor agonist which produces sedation and superficial and visceral analgesia which is dose dependent in its depth and duration. Profound lethargy and a characteristic lowering of the head with reduced sensitivity to environmental stimuli (sounds, etc.) are seen with detomidine. A short period of incoordination is characteristically followed by immobility and a firm stance with front legs well spread. The analgesic effect is most readily seen as an increase in the pain threshold at the body surface. With intravenous administration, both superficial and visceral analgesia are seen to depths which allow for such procedures as flank incision after local anesthesia, penetration of the peritoneum, and rectal examinations for diagnosis of colic. Sensitivity to touch is little affected and in some cases may actually be enhanced.Chemical restraint and pain relief provided by α-2 adrenoceptor agonists facilitates the handling of fractious animals, and aids in the conduct of uncomfortable diagnostic or therapeutic procedures such as endoscopy, nasogastric tubing, and rectal examinations. It also facilitates surgical procedures (with or without local anesthesia) such as suturing of skin lacerations, flank incisions for ovariectomy, vaginal suturing, and castration. With detomidine administration, heart rate is markedly decreased, blood pressure is initially elevated and then a steady decline to normal is seen. A transient change in the conductivity of the cardiac muscle may occur, as evidenced by partial atrioventricular (AV) and sinoauricular (SA) blocks. This change in the conductivity of the cardiac muscle may be prevented by I.V. administration of atropine at a dosage of 0.02 mg/kg of body weight. A diuretic effect is usually observed within 45 to 60 minutes after treatment. Effects on blood clotting time or other hematological parameters was not encountered at dosages of 20 or 40 µg per kg of body weight. Respiratory responses include an initial slowing of respiration within a few seconds to 1 to 2 minutes after administration, increasing to normal within 5 minutes. An initial decrease in tidal volume is followed by an increase.
Post-approval experience: Although all adverse reactions are not reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in under-reporting. The adverse events here reflect reported observations regardless of association with causality. Adverse events are listed by body system, in descending order of frequency:
Systemic disorders: Lethargy, hyperthermia
Neurological disorders: Ataxia, sedation and muscular tremors, loss of consciousness
Respiratory tract disorders: Tachypnea, dyspnea
Cardiovascular disorders: Tachycardia
Immune system disorders: Urticaria
Digestive tract disorders: Abdominal pain
Skin and appendages disorders: Hyperhidrosis
Storage
15°-30°C (59°-86°F) - Protect from light. Content should be used within 90 days of the first puncture (20 punctures maximum).Presentation: Supplied in 5 mL and 20 mL multidose vials containing 10 mg detomidine hydrochloride per mL.
Manufactured for:
Modern Veterinary Therapeutics, LLC, Sunrise, Florida 33326 USA
Tel. (888) 590 9839
info@modernveterinarytherapeutics.com
www.modernveterinarytherapeutics.com
Imported by:
Modern Veterinary Therapeutics Inc., 261065 Wagon Wheel Way, Bay 3, Balzac (Rocky View County), AB T4A 0T5
Orders & Product information: Call 1 888 590 9839
Made in The Netherlands.
Revision Date: 5 June 2024
CPN: 1354002.8
261065 WAGON WHEEL WAY, ROCKY VIEW COUNTY, AB, T4A 0T5
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
