Canalevia-CA1
This treatment applies to the following species: Company: Jaguar Animal Health
Company: Jaguar Animal Health
(crofelemer delayed-release tablets)
125 mg
antidiarrheal
For oral use in dogs only
For treatment of chemotherapy-induced diarrhea in dogs.
Canalevia-CA1 Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed. It is a violation of Federal law to use this product other than as directed in the labeling.
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-552
Description
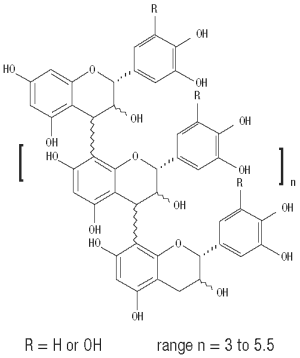
CANALEVIA-CA1 (crofelemer delayed-release tablets) is an antidiarrheal, enteric-coated drug product for oral administration. CANALEVIA-CA1 containing 125 mg of crofelemer, a botanical drug substance that is derived from the red latex of Croton lechleri Müll. Arg. Crofelemer is an oligomeric proanthocyanidin mixture primarily composed of (+)-catechin, (-)-epicatechin, (+)-gallocatechin, and (-)-epigallocatechin monomer units linked in random sequence, as represented below. The average degree of polymerization for the oligomers ranges between 5 and 7.5, as determined by phloroglucinol degradation.
INDICATION: CANALEVIA-CA1 is indicated for the treatment of chemotherapy-induced diarrhea in dogs.
Dosage and Administration
Administer 1 tablet orally twice daily for 3 days for dogs weighing ≤ 140 pounds. Administer 2 tablets orally twice daily for 3 days for dogs weighing >140 pounds. Tablets should not be broken, crushed, or chewed. If the tablet is chewed, one additional dose may be administered. Give with or without food.Contraindications
Do not use in patients with a known hypersensitivity to crofelemer.
Warnings
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans.Precautions
The safe use of CANALEVIA-CA1 has not been evaluated in dogs that are pregnant, lactating, or intended for breeding.Prior to using CANALEVIA-CA1, rule out infectious etiologies of diarrhea. Prescribing CANALEVIA-CA1 to dogs with diarrhea secondary to infectious etiologies may delay appropriate treatment.
Adverse Reactions
In a masked, randomized study to support a reasonable expectation of effectiveness (CANA-001a) there was similar incidence of adverse events in dogs administered crofelemer (not commercial formulation) compared to control dogs administered a vehicle control. Crofelemer was administered orally twice a day for three days as enteric-coated beads containing 40 mg of the active ingredient. The vehicle control was enteric-coated beads without the active ingredient. The dogs had daily assessments for changes in attitude, hydration, appetite, and abdominal palpation. Table 1 shows the adverse reactions during CANA-001a study.Table 1. Adverse Reactions in CANA-001a Study to Support Reasonable Expectation of Effectiveness
|
Adverse Reaction1 |
Crofelemer N = 29 |
Vehicle Control N = 32 |
|
Appetite decreased |
7 (24%) |
9 (28%) |
|
Attitude depressed |
5 (17%) |
5 (16%) |
|
Activity decreased |
4 (14%) |
4 (13%) |
|
Dehydration2 |
4 (14%) |
5 (16%) |
|
Abdominal palpation indicated pain3 |
3 (10%) |
5 (16%) |
|
Vomiting |
1 (3%) |
2 (6%) |
1Dogs may have experienced more than one occurrence and more than one type of abnormal assessment during the study
2Two crofelemer treated dogs and one vehicle control dog were administered fluid therapy
3One of the vehicle control dogs was reported to not allow abdominal palpation at one timepoint on Day 2
In an open-label pilot study (CANA-001b) 48 dogs were administered crofelemer (not commercial formulation) orally twice a day for three days. The adverse reactions included decreased appetite in 16/48 (33%) dogs, vomiting in 3 (6%) dogs, decreased activity in 3 dogs (6%), abdominal pain in 1 (2%) dog, and 1 (2%) dog had blood in feces.
In a separate pilot clinical field study (CANA-003), dogs with acute diarrhea were randomized to receive crofelemer or placebo tablets. The study included 22 dogs administered crofelemer (not commercial formulation) tablets and 112 dogs administered 125 mg CANALEVIA-CA1 tablets. There were 66 dogs who received placebo tablets. Dogs were administered tablets orally twice a day for three days. Hematology and clinical chemistry were obtained prior to dosing and on Day 3 at the end of the study. Table 2 shows adverse reactions during the study.
Table 2. Adverse Reactions in Pilot Field Study CANA-003
|
Adverse Reaction |
Crofelemer N=134 |
Placebo N=66 |
|
Vomiting |
11 (8%) |
1 (2%) |
|
Decreased blood glucose1 |
10 (8%) |
3 (5%) |
|
Urinary system abnormality2 |
10 (8%) |
2 (3%) |
|
Upper respiratory signs3 |
7 (5%) |
0 (0%) |
|
Decreased blood calcium4 |
5 (4%) |
0 (0%) |
1Dogs with decreased blood glucose at study end had normal blood glucose (reference range 74-145 mg/dL) prior to dosing. None of the dogs were reported to be clinically affected by the decreased blood glucose. The low blood glucose values ranged from 34 to 71 mg/dL.
2Urinary system abnormalities were documented as development of a urinary tract infection, cystitis, or a worsening pyuria in the urine.
3Upper respiratory signs included coughing, nasal discharge, sneezing, and congestion.
4Dogs with decreased serum calcium (reference range 8.7-12 mg/dL) at study end had normal calcium at screening. The decreased calcium values were not associated with decreased albumin values. None of the dogs were reported to be clinically affected by the decreased serum calcium. The lowest reported calcium was 5.8 mg/dL.
Five dogs administered crofelemer and one placebo group dog were removed from the study for no improvement in stools. One additional dog administered crofelemer was removed for hemorrhagic stool.
CONTACT INFORMATION: To report suspected adverse drug events and/or to obtain a copy of the Safety Data Sheet (SDS) or for technical assistance, call Jaguar Animal Health at 1-877-787-3001.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Clinical Pharmacology
Mechanism of Action: In humans, crofelemer is an inhibitor of both the cyclic adenosine monophosphate (cAMP)-stimulated cystic fibrosis transmembrane conductance regulator (CFTR) chloride ion (Cl-) channel, and the calcium-activated Cl- channels (CaCC) at the luminal membrane of enterocytes. The CFTR Cl- channel and CaCC regulate Cl- and the osmotic gradient which causes fluid influx into the lumen. Crofelemer inhibits the hypersecretion of Cl- in diarrhea and normalizes the fluid influx into the GI tract. The mechanism of action in the dog has not been fully characterized.REASONABLE EXPECTATION OF EFFECTIVENESS: A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
CANALEVIA-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found at www.fda.gov/animalca.
A reasonable expectation of effectiveness for CANALEVIA-CA1 is based on a pilot field study.
A multi-site, randomized, masked, vehicle-controlled field study in dogs with diarrhea of various etiologies was conducted to assess the effectiveness of crofelemer (not commercial formulation). The effectiveness dataset included 12 dogs administered crofelemer and 12 dogs administered vehicle control. Dogs were between the ages of four months and 12 years with a fecal score of 4 or 5 (watery or liquid stools with little or no particulate matter or severe watery diarrhea with no particulate matter visible). Crofelemer was administered at a dose of 1.9 to 12.9 mg/kg every 12 hours for three days. Fecal scores were obtained every four hours during the three day study. Fecal scores were based on a modified version of the Purina Fecal Scoring System and were defined as:
1. Well-formed stools with slightly moist surface which leaves marks when picked up
2. Soft or very soft, moist, amorphous
3. Viscous liquid with some particulate matter
4. Watery, liquid stools with little particulate matter visible
5. Severe watery diarrhea, no particulate matter visible
6. Hemorrhagic diarrhea
The resolution of diarrhea was considered treatment success and was defined as a fecal score of 1 or 2. After a dog achieved resolution of diarrhea during the 3 day study, subsequent fecal scores had to be maintained as a 1 or 2 in order to be considered a treatment success.
The resolution of diarrhea per day (24 hour treatment block) is described in Table 3.
Table 3. Number of Dogs Achieving Resolution of Diarrhea per 24 Hour Treatment Block
|
Treatment block |
Crofelemer N=12 |
Vehicle Control N=12 |
|
≤24 hours |
2 (17%) |
0 (0%) |
|
>24 hours and ≤48 hours |
2 (17%) |
0 (0%) |
|
>48 hours and ≤72 hours |
5 (42%) |
3 (25%) |
Daily assessments included body temperature; fecal scores; and a scored assessment of general attitude, hydration status, appetite, and reaction to abdominal palpation (see ADVERSE REACTIONS).
TARGET ANIMAL SAFETY:
The safety of CANALEVIA-CA1 and crofelemer was evaluated in four laboratory studies.
Target Animal Safety Study: In a 9 day laboratory safety study, healthy four month old Beagle dogs were orally administered crofelemer at 0, 27.2, 54.3, or 162.9 mg/kg twice daily (4 dogs per sex in each group) for 9 days. All dogs survived to study termination.
Clinical observations related to CANALEVIA-CA1 included discolored red feces, watery feces, and vomiting in a dose-dependent manner with increased incidence in the higher dose groups.
Hematology findings related to CANALEVIA-CA1 included 3 dogs that had a decreased hematocrit (reference range 37.2-52%) at study end compared to acclimation: 1 male administered 27.2 mg/kg with hematocrit of 39.6% prior to dosing and 35.8% on Day 9; 1 female administered 162.9 mg/kg with hematocrit of 40.7% prior to dosing and 36.4% on Day 9; and 1 female administered 162.9 mg/kg with hematocrit of 36.4% prior to dosing and 36.1% on Day 9.
There were no clinically relevant or significant findings for body weight, physical examination, food consumption, electrocardiogram, ophthalmoscopic, or necropsy examinations.
Target Animal Toxicity Studies: In two separate laboratory studies, healthy six to seven month old Beagle dogs were orally administered crofelemer (not commercial formulation) at 0, 50, 175, or 600 mg/kg once daily (four dogs per sex in each group) for 30 days and nine months duration, respectively. All dogs survived to study termination.
Clinical observations related to crofelemer included abnormal feces (mainly black and rust colored mucoid feces and diarrhea) observed as soon as 1 day after the first administration and occurred in a dose-dependent manner with increased incidence in the higher dose groups. Dogs in the 600 mg/kg/day group did not gain weight at the same rate, had decreased food consumption, and higher incidences of vomiting compared to the other groups.
Hematology findings related to crofelemer at the dose of 600 mg/kg/day were consistent with a microcytic, hypochromic anemia with mild regeneration, and thrombocytosis as characterized by decreases in hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration; and increases in platelets, percent reticulocytes, and absolute reticulocytes compared to the other groups. Females at 175 mg/kg/day and males and females at 600 mg/kg/day had increased neutrophils compared to the other groups. Crofelemer-related serum chemistry findings at 600 mg/kg/day, and to a lesser severity at 175 mg/kg/day, included decreased albumin, total protein, cholesterol, calcium, and sodium and increased potassium compared to the other groups.
On necropsy, gross lesions related to crofelemer included localized irritation of the gastrointestinal tract (reddened areas, erosion, and red streaks) and discoloration of the gastrointestinal tract (dark red areas and gray areas) and lymph nodes (red, gray, and green). Gross lesions were observed at all crofelemer doses in a dose-dependent manner where higher doses had higher incidences.
On histopathology, crofelemer-related lesions included congestion in the small and large intestines; inflammation and pigmentation of the gastrointestinal tract and pigmentation in the liver; congestion in the mesenteric and suprapharyngeal lymph nodes; histiocytosis, erythrocytosis, basophilic bodies, and pigmentation of the lymph nodes; and splenic macrophage infiltration. Histopathologic lesions occurred in a dose-dependent manner where the higher doses had higher incidences.
Cardiovascular Study: The cardiovascular effects of crofelemer (not commercial formulation) were evaluated in a laboratory study in four healthy one to one and a half years old male Beagle dogs implanted with radiotelemetry devices. Dogs were orally administered crofelemer at 0, 60, 200, and 600 mg/kg one time. Each dog received all treatments with a seven day washout period between treatments. There were no crofelemer-related effects on the cardiovascular parameters (heart rate, blood pressure, electrocardiograms). Clinical observations related to crofelemer included soft, watery, mucoid, or discolored (brown or red) feces in the 200 and 600 mg/kg groups.
STORAGE INFORMATION: Store at 20°C-25°C (68°F-77°F) with excursions permitted between 15°C-30°C (59°F-86°F).
How Supplied
CANALEVIA-CA1 tablets are available as white, unscored enteric-coated tablets containing 125mg of crofelemer packaged in bottles containing 60 tablets.Distributed by:
Jaguar Animal Health, San Francisco, CA 94104
70042882
JAH 111-060 Rev 11/21
|
Net contents: |
NDC |
|
60 tablets |
86149-111-60 |
CPN: 1868002.0
200 PINE ST., SUITE 600, SAN FRANCISCO, CA, 94104
| Telephone: | 415-371-8310 | |
| Website: | www.jaguar.health | |
| Email: | hello@jaguar.health |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
