BRAVECTO Topical Solution for Dogs 500 mg (Canada)
This treatment applies to the following species: Company: Merck Animal Health
Company: Merck Animal Health
Fluralaner Topical Solution, 112.5 mg, 250 mg, 500 mg, 1000 mg, 1400 mg
Veterinary Use Only
DIN 02454947, 02454955, 02454963, 02454971, 02454998
Description
BRAVECTO (Fluralaner Topical Solution) contains 280 mg/mL (28%) of fluralaner. Each tube is formulated to provide a minimum dose of 25 mg/kg body weight.
Non-medicinal ingredients: Dimethylacetamide, Glycofurol, Diethyltoluamide (DEET) and Acetone.
THERAPEUTIC CLASSIFICATION:
Antiparasitic
BRAVECTO Topical Solution for Dogs 500 mg Indications
BRAVECTO kills fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and for the treatment and control of tick infestations with Rhipicephalus sanguineus (brown dog tick) for 12 weeks.
BRAVECTO is also indicated as an aid in the treatment and control of tick infestations with Ixodes scapularis (black-legged tick) and Dermacentor variabilis (American dog tick) for 12 weeks.
BRAVECTO is indicated for dogs and puppies 6 months of age and older and weighing 2 kg or greater.
Dosage and Administration
BRAVECTO should be administered topically as a single dose every 12 weeks according to the Dosage Schedule below to provide a minimum dose of 25 mg/kg body weight.
Dosage Schedule
|
Body Weight Ranges (kg) |
Fluralaner content (mg/tube) |
Tubes Administered |
Package Colour |
|
2 - 4.5 |
112.5 |
One |
Yellow |
|
> 4.5 - 10 |
250 |
One |
Orange |
|
> 10 - 20 |
500 |
One |
Green |
|
> 20 - 40 |
1000 |
One |
Blue |
|
> 40 - 56* |
1400 |
One |
Pink |
* Dogs over 56 kg should be administered the appropriate combination of tubes.
Step 1: Immediately before use, open the pouch and remove the tube.
Put on gloves provided at the point of sale.
Hold the tube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counter clockwise one full turn.
DO NOT REMOVE THE CAP.
The cap is designed to stay on the tube for dosing.
The tube is open and ready for application when a breaking of the seal is felt.
Step 2: The dog should be standing or lying with its back horizontal during application. Part the hair at the administration site.
Place the tube tip vertically against the skin between the shoulder blades of the dog.
Step 3: Squeeze the tube and gently apply BRAVECTO in one or more spots starting between the shoulder blades and continuing along the dog’s back.
Avoid applying an excessive amount of solution in any one spot which may cause some solution to run or drip off of the dog.
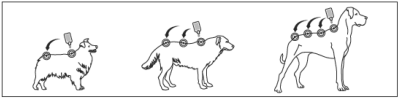
Bathing or water immersion 3 days or more after administration will not reduce the effectiveness of BRAVECTO (see EFFICACY).
Treatment with BRAVECTO may begin at any time of the year. Due to climate variations across the country, Canada has highly variable distributions and abundance of flea and tick infestations. A comprehensive plan, based on regional risk assessment is recommended to determine an appropriate dosing interval.
Contraindications
This product should not be given to dogs with known or suspected allergy or intolerance to fluralaner or the non-medicinal ingredients in this product.
CAUTIONS:
Avoid ingestion (see ANIMAL SAFETY).
BRAVECTO has not been shown to be effective for 12-weeks’ duration in puppies less than 6 months of age.
Fluralaner is a member of the isoxazoline class. This class has been associated with neurological adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurological disorders.
Warnings
● Keep out of the reach of children.
● Do not contact or allow children to contact the application site until it is no longer noticeable on the animal’s skin/fur. This includes cuddling and sharing a bed with the animal. It takes up to 48 hours for the application site to become dry, but it will be noticeable for longer.
● This product is very sticky and binds to skin and may also bind to surfaces after spillage of the product.
● Special precautions should be taken by the person who administers this product.
Adverse reactions have been reported in a small number of people after skin contact. Avoid contact with skin. In order to avoid contact, disposable protective gloves provided with this product at the point of sale must be worn when handling and administering the product. If accidental skin contact occurs, wash the skin thoroughly with soap and water immediately. In some cases, soap and water are not sufficient to remove the product, therefore gloves must be worn. Wash hands after use of the product.
● This product can be harmful if ingested. If accidental ingestion occurs, seek medical advice. In order to prevent children from getting direct access to the product, keep tube(s) in unopened pouch until use. Keep unopened pouches in the original carton between uses. Dispose of used tubes immediately after use.
● Do not eat, drink or smoke while handling the product.
● This product can cause eye irritation. If accidental contact occurs, flush eyes thoroughly with water.
● Hypersensitivity reactions to the product have been reported in a small number of people. The product should not be used by persons with a hypersensitivity to the active substance or to any of the non-medicinal ingredients (See DESCRIPTION for active substance and list of non-medicinal ingredients).
● The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
Adverse Reactions
In a well-controlled U.S. field study, which included a total of 165 households and 321 treated dogs (221 with fluralaner and 100 with a topical active control), all potential adverse reactions were recorded and the most frequently reported are listed in the table below:
Percentage of Dogs with Adverse Reactions in the Field Study
|
Adverse Reaction (AR) |
BRAVECTO Group: Percentage of Dogs with the AR During the 105-Day Study (n= 221 dogs) |
Active Control Group: Percentage of Dogs with the AR During the 84-Day Study (n= 100 dogs) |
|
Vomiting |
6.3% |
6.0% |
|
Alopecia |
4.1% |
2.0% |
|
Diarrhea |
2.7% |
11.0% |
|
Lethargy |
2.7% |
2.0% |
|
Decreased Appetite |
1.4% |
0.0% |
|
Moist Dermatitis/Rash |
0.9% |
0.0% |
In this field study, the reported serious adverse reactions were not associated with the treatments. Two (2) dogs treated with BRAVECTO with no prior history of seizures each experienced a seizure. One dog had two seizures a day apart about 18 days after its first dose. The dog was started on antiepileptic medication and had no additional seizures during the study. A second dog had a seizure 76 days after its first dose and 3 days after starting fluoxetine for separation anxiety. The fluoxetine was discontinued and the dog experienced no additional seizures during the study. A third dog treated with BRAVECTO topical solution was observed by the owner to be off balance for about 30 minutes five days after its first dose and had no similar observations after the second dose. One dog with a history of seizures had a seizure the day after the second dose of the active control.
In two well-controlled laboratory dose confirmation studies, one dog developed mild to moderate redness, flaking, crusts/scabs and alopecia at the treatment site from Day 1 through 14 after application of BRAVECTO on Day 0, and one dog developed self-limiting generalized erythema (possible allergic reaction) one day after treatment with BRAVECTO.
In a European field study conducted in 474 cats receiving topical fluralaner, there were three reports of facial dermatitis in humans after close contact with the application site within 4 days of application. Association with the treatment was not substantiated in two cases and in the third case, close contact occurred before the skin at the application site was dry. Such signs were not reported in the US and European field studies in dogs.
Clinical Pharmacology
Peak fluralaner concentrations are achieved between 7 and 42 days following topical administration and the elimination half-life ranges between 14 and 29 days. The bioavailability of fluralaner following topical administration is approximately 25%.
MODE OF ACTION:
Fluralaner has a systemic mode of action and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
EFFICACY:
In well-controlled U.S. laboratory studies, BRAVECTO killed fleas and reduced the numbers of live fleas on dogs by ≥ 98.7% and 100% within 24 and 48 hours respectively, post-infestation for 12 weeks.
In well-controlled laboratory studies, BRAVECTO demonstrated ≥ 92.7% effectiveness against Rhipicephalus sanguineus and ≥ 86.6% effectiveness against Ixodes scapularis and Dermacentor variabilis 48 hours post-infestation for 12 weeks.
In one study conducted with Ixodes scapularis and in one study conducted with Dermacentor variabilis, the onset of immediate efficacy (≥ 90% by Day 2) was reached later than Day 2. This may be attributable to variability in the topical absorption rate between individual dogs.
Efficacy of BRAVECTO topical solution against fleas and ticks in laboratory studies 48 hours post-infestation
|
|
Study |
% Efficacy* |
|||
|
Day 2 |
Day 30 |
Day 58 |
Day 86 |
||
|
Ctenocephalides felis |
1 |
100 (100) |
100 (100) |
100 (100) |
100 (100) |
|
Dermacentor variabilis |
1 |
100 (100) |
100 (100) |
100 (100) |
100 (100) |
|
2 |
86.7 (97.5) |
100 (100) |
100 (100) |
97.3 (98.0) |
|
|
Ixodes scapularis |
1 |
91.6 (96.6) |
100 (100) |
100 (100) |
100 (100) |
|
2 |
86.6 (93.3) |
98.0 (99.1) |
99.4 (99.5) |
97.2 (98.2) |
|
|
Rhipicephalus sanguineus |
1 |
99.7 (99.8) |
99.6 (99.7) |
99.5 (99.6) |
100 (100) |
|
2 |
92.7 (97.6) |
100 (100) |
98.5 (98.9) |
94.7 (96.7) |
|
|
3 |
92.8 (96.6) |
100 (100) |
100 (100) |
99.6 (99.7) |
|
* arithmetic mean (geometric mean). Note: the arithmetic mean was used to assess efficacy.
In a well-controlled U.S. field study, a single dose of BRAVECTO reduced fleas by ≥ 99.8% for 12 weeks. Dogs with signs of flea allergy dermatitis showed improvement in erythema, alopecia, papules, scales, crusts, and excoriation as a direct result of eliminating flea infestations.
In a European laboratory study, BRAVECTO demonstrated > 98.4% efficacy against fleas and Ixodes ricinus ticks after multiple water immersion or shampooing occasions, the first occurring 3 days after administration, for up to 12 weeks.
ANIMAL SAFETY:
Margin of Safety Study: In a margin of safety study, BRAVECTO was administered topically to 8- to 9-week-old-puppies at 1, 3, and 5X the maximum labeled dose of 56 mg/kg at three, 8-week intervals (8 dogs per group). The dogs in the control group (0X) were administered mineral oil.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Cosmetic changes at the application site included matting/clumping/spiking of hair, wetness, or a greasy appearance.
Oral Safety Study: In a safety study, one dose of BRAVECTO topical solution was administered orally to 8- to 10-month-old-puppies at 1X the maximum labeled dose of 56 mg/kg. The dogs in the control group (0X) were administered saline orally.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Five of the six treated dogs experienced salivation immediately after administration. One treated animal experienced loose feces with blood three hours after treatment. One treated animal experienced vomiting eight hours after administration.
Reproductive Safety: Reproductive safety was evaluated with the oral chewable tablet formulation of fluralaner. This product was administered orally to intact, reproductively-sound male and female Beagles at a dose of up to 168 mg/kg (equivalent to 3X the maximum treatment dose) on three to four occasions at 8-week intervals. The dogs in the control group (0X) were untreated.
There were no clinically-relevant, treatment-related effects on the body weights, food consumption, reproductive performance, semen analysis, litter data, gross necropsy (adult dogs) or histopathology findings (adult dogs and puppies). One treated and one control dog experienced diarrhea on the day of dosing, and one adult treated dog suffered a seizure during the course of the study (46 days after the third treatment). Abnormal salivation was observed on 17 occasions: in six treated dogs (11 occasions) after dosing and four control dogs (6 occasions).
The following abnormalities were noted in 7 pups from 2 of the 10 dams in only the treated group during gross necropsy examination: limb deformity (4 pups), enlarged heart (2 pups), enlarged spleen (3 pups), and cleft palate (2 pups). During veterinary examination at Week 7, two pups from the control group had inguinal testicles, and two and four pups from the treated group had inguinal and cryptorchid testicles, respectively. No undescended testicles were observed at the time of necropsy (days 50 to 71).
In a well-controlled field study BRAVECTO was used concurrently with other medications, such as vaccines, anthelmintics, antibiotics, steroids and sedatives. No adverse reactions were observed from the concurrent use of BRAVECTO with other medications.
STORAGE CONDITIONS:
Do not store above 30°C. Protect from freezing.
How Supplied
BRAVECTO (Fluralaner Topical Solution) is available in five strengths for use in dogs (112.5 mg, 250 mg, 500 mg, 1000 mg, and 1400 mg fluralaner per tube). Each tube is packaged individually in a pouch. Product may be supplied in 1 or 2 tubes per box.
Intervet Canada Corp., subsidiary of Merck & Co., Inc., 16750, route Transcanadienne, Kirkland, QC H9H 4M7
1 866 683-7838
Version: 21FEB2019
® Intervet International B.V. Used under license.
CPN: 1208298.2
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
