BOVILIS Once PMH IN (Canada)
This treatment applies to the following species:Mannheimia Haemolytica-Pasteurella Multocida Vaccine, Avirulent Live Culture
Cattle Vaccine
For Intranasal Use
BOVILIS Once PMH IN Indications
This product has been shown effective for the vaccination of healthy cattle 1 week of age or older against disease due to Mannheimia haemolytica and Pasteurella multocida. Duration of immunity has not been established. For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov.
MIXING DIRECTIONS: Rehydrate freeze dried vial with accompanying vial of diluent. Mix reconstituted vial well.
DOSAGE AND ROUTE: Administer a single 1 mL dose aseptically into each nostril or 2 mL into only one nostril. Historically, annual revaccination with this product has been recommended. The need for this booster has not been established. For more information on revaccination frequency, consult your veterinarian.
Mixing Instructions (for 100 mL/50 doses format)
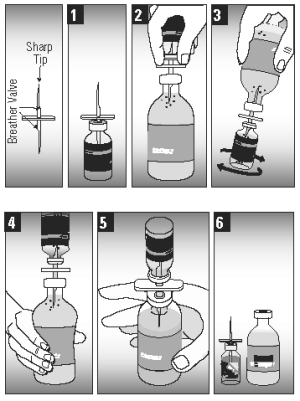
CAUTION: THE TRANSFER NEEDLE, INCLUDED IN THE CARTON PACKAGING (50 DOSES), IS SHARP AND MAY CAUSE INJURY TO SELF OR ANIMALS IF NOT HANDLED OR DISPOSED OF PROPERLY.
1. Insert the transfer needle fully into the small vial of dehydrated vaccine to release the vacuum.
2. Insert the other end of the transfer needle fully into the large vial of sterile diluent.
3. Squeeze enough diluent from the large bottle into the smaller vial to rehydrate the vaccine.
4. With the two bottles still attached, swirl the small vial gently until the vaccine is mixed.
5. With the small vial upside down, squeeze the large bottle several times to draw the mixed vaccine into the large bottle.
6. Separate the bottles and remove the vaccine label from the small vial and apply it to the large bottle for proper identification.
CAUTIONS: Store at 2 to 8°C (35 to 46°F). Do not freeze. Use entire contents when first opened. Do not mix with other products, except as specified on the label. Inactivate unused contents before disposal. Do not vaccinate within 21 days of slaughter. This product has not been tested in pregnant animals. Anaphylactoid reactions may occur following use. Antidote: Epinephrine. Contains streptomycin as a preservative. In case of human exposure, contact a physician.
FOR ANIMAL USE ONLY
Intervet Inc. d/b/a Merck Animal Health, Omaha, NE 68103, USA
VLN 165A / PCN 1861.02
For patent information:
http://www.merck.com/product/patent/home.html
1 866 683-7838
|
|
|
|
Code |
|
|
20 mL |
10 Doses |
2 mL per dose |
139541 |
167421 R3 |
|
25 x 1 Dose / 2 mL |
25 Doses |
158939 |
166683 R3 |
|
|
100 mL |
50 Doses |
138911 |
167679 R6 |
CPN: 1208341.0
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
