Bovikalc (Canada)
This treatment applies to the following species:Veterinary Health Product
ORAL CALCIUM SUPPLEMENT
FOR FRESHENING COWS
Warnings
No withdrawal period is required when used according to the label. Keep out of reach of children.NN.S0Z9
FAST & SUSTAINED RELEASE OF CALCIUM
Bovikalc® is an oral calcium supplement for use around parturition in fresh dairy cows.
Direction for Use:
Minimum Protocol: Give one bolus immediately after calving and a second bolus 12 - 24 hours after calving. Only administer using the Bovikalc® branded applicator.
Extended Protocol: For high yielding cows administer:
● 1st bolus at the first signs of parturition
● 2nd bolus immediately after calving
● 3rd bolus 12 - 24 hours after calving
● 4th bolus 24 - 48 hours after calving
Please discuss protocol with your veterinarian before implementation.
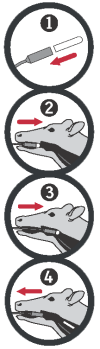
To remove the bolus from the plastic tube, open the lid, invert the tube, and carefully slide the bolus out into your hand. Place the bolus in the bolus applicator with the rounded end pointing outward as shown in image 1. If right-handed, place yourself at the cow’s right side.
Grab the cow’s upper jaw with your left hand. Keep the cow’s head at a natural position and a little towards yourself. Gently guide the loaded Bovikalc® applicator into the cow’s mouth.
Gently guide the applicator over the tongue. When the applicator is behind the tongue, squeeze the handle to release the bolus. To assist passage and to stimulate the swallowing reflex, gently stroke the hard palate ridges. Ensure constant access to fresh water for the cow.
Wait a moment for the cow to swallow and then remove the applicator. A bolus may block the airway if not properly administered or if broken or not swallowed. Look for signs of breathing difficulty for 5 minutes after administering the bolus. Make sure to wash and dry the applicator after each use.
Active Ingredients per bolus:
Calcium chloride - 579.1 mg/g, Calcium sulfate - 231.6 mg/g
Inactive Ingredients
Water, Acetylated monoglycerides, Xanthan gum
Cautions:
Do not use in cows for initial treatment of milk fever, with clinical signs of milk fever or laying cows.
Do not use broken or damaged boluses.
Do not use excessive force when administering to the cow.
Cows should be properly restrained for bolus administration.
Do not administer to cows without a normal swallowing reflex.
This VHP may not be incorporated into a mixed feed.
Store at or below 30°C. Boluses are sensitive to humidity and should be stored in the plastic tubes until immediately before use.
Bovikalc® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
Attention! Do not use broken or damaged boluses.
Notifier’s name:
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
Reaction Reporting:
1-877-565-5501
Manufactured by:
Boehringer Ingelheim Animal Health Denmark A/S
Reg. No.: 208-R854833
Denmark
Product Information
1-800-263-5103
www.boehringer-ingelheim.ca
MADE IN DENMARK
GTIN: (01)05703655003833
|
Net Contents: |
|
|
4 x 192 g BOLUSES |
125887-004 |
CPN: 1182208.0
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
