The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
AmTech Glycopyrrolate Injectable
This page contains information on AmTech Glycopyrrolate Injectable for veterinary use.The information provided typically includes the following:
- AmTech Glycopyrrolate Injectable Indications
- Warnings and cautions for AmTech Glycopyrrolate Injectable
- Direction and dosage information for AmTech Glycopyrrolate Injectable
Amtech Glycopyrrolate Injectable
This treatment applies to the following species:0.2 Mg/ml
ANADA 200-365, Approved by FDA
For Preanesthetic Anticholinergic Use in Dogs and Cats Only
AmTech Glycopyrrolate Injectable Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Each 1 mL contains:
|
Glycopyrrolate |
0.2 mg |
|
Benzyl alcohol (preservative) |
0.9% |
|
Water for injection, USP |
q.s. |
pH adjusted, when necessary, with hydrochloric acid and/or sodium hydroxide.
For intramuscular, intravenous or subcutaneous use in dogs and intramuscular use in cats.
Glycopyrrolate Injectable is a synthetic anticholinergic agent. It is a quaternary ammonium compound with the following chemical structure:
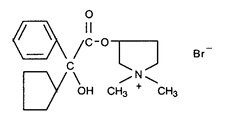
Glycopyrrolate Injectable is a clear colorless liquid.
Pharmacology
Glycopyrrolate, like other anticholinergic agents, inhibits the action of acetylcholine on structures innervated by post-ganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine that lack cholinergic innervation. In the dog it diminishes the volume and free acidity of gastric secretions and reduces intestinal motility. It diminishes and controls excessive pharyngeal, tracheal and bronchial secretions and has a longer lasting effect than atropine. In the cat it controls excessive salivation and pharyngeal secretions.
In anesthetized dogs intravenous doses of 0.0023 to 0.0045 mg/lb markedly reduced intestinal tone and moderately inhibited amplitude of intestinal contraction but had essentially no effect on respirations, periodic arterial blood pressure or cardiac rate. These doses of glycopyrrolate reduced bradycardia, hypertension and the intestinal hyperactivity resulting from peripheral vagal stimulation.
In dog studies glycopyrrolate antagonized muscarinic symptoms (e.g., bronchorrhea, bronchospasm, bradycardia and intestinal hypermotility) induced by cholinergic drugs such as the anticholinesterases, affording the same protection as atropine against bradycardia.
The intestinal hyperactivity and copious salivation produced by a subcutaneous dose of methacholine chloride, 0.5 mg/kg, were suppressed by glycopyrrolate in intravenous doses as low as 0.0045 mg/lb in the dog.
In a study1 glycopyrrolate at 0.004 and 0.008 mg/lb had a longer lasting effect and more smooth-muscle relaxation, with little adverse cardiovascular effect when compared to atropine at 0.02 mg/lb. Glycopyrrolate was effective in preventing aspiration of gastric secretions and the resulting pulmonary complications, not only by producing a higher pH of gastric secretions but in the reduction of intestinal smooth-muscle activity and thus the likelihood of regurgitation.
The polar ammonium moiety of glycopyrrolate limits its passage across the lipid membranes such as the blood-brain barrier in contrast to the belladonna alkaloids which are non-polar tertiary amines.
In a cat study, 14C-labeled glycopyrrolate was administered intramuscularly at doses ranging from 0.018 to 0.024 mg/kg (0.008 to 0.011 mg/lb). Peak blood levels of radioactivity were detected at 15 minutes following injections. Blood levels of radioactivity declined slowly during a 48-hour period with the drug being excreted approximately equally between urinary and fecal excretion routes.
In dog studies peak effect occurred approximately 30 to 45 minutes after subcutaneous or intramuscular administration. The vagal blocking effect persisted for two to three hours and the antisialagogue effect persisted for up to seven hours, periods longer than for atropine. With intravenous injection the onset of action was generally evident within one minute.
Toxicology
Acute and chronic toxicity studies have shown glycopyrrolate to have a low order of toxicity.
In the dog the LD50 for intravenous administration is 25 mg/kg and daily intravenous doses of either 0.4 or 2 mg/kg five days per week for four consecutive weeks revealed no signs of toxicity. The oral feeding of high levels (27 mg/kg) of glycopyrrolate for seven weeks produced no signs of toxicity in the dog.
In the cat the LD50 for intramuscular administration is 283 mg/kg. In a ten-week study cats received daily intramuscular doses of 0.01, 0.03 and 0.05 mg/kg. No changes considered to be related to glycopyrrolate were seen in food consumption, hematology, biochemical, urinalysis or gross pathology. Histopathology showed slight to moderate proliferation of intrahepatic bile ducts at the 0.05 mg/kg/day dose level.
AmTech Glycopyrrolate Injectable Indications
Glycopyrrolate Injectable is indicated as a preanesthetic anticholinergic agent in dogs and cats.
In dogs it reduces salivary, tracheobronchial and pharyngeal secretions, reduces the volume and free acidity of gastric secretion, and blocks cardiac vagal inhibitory reflexes during induction of anesthesia and intubation.
In cats it reduces salivary and pharyngeal secretions.
Precautions
There are no absolute contraindications to the use of Glycopyrrolate Injectable in conjunction with anesthesia except known hypersensitivity to glycopyrrolate. Reproduction studies in rats and rabbits revealed no teratogenic effects from glycopyrrolate; however, the anticholinergic action in this agent resulted in diminished rates of conception and of survival at weaning in rats in a dose-related manner. Reproduction studies have not been conducted on glycopyrrolate in dogs and cats. Therefore, Glycopyrrolate Injectable should not be administered to pregnant bitches or queens. The excretion of Glycopyrrolate Injectable may be prolonged in animals with impaired renal function or impaired gastrointestinal function.
Side Effects
Mild mydriasis, xerostomia and tachycardia may be seen with Glycopyrrolate Injectable. These are extensions of the fundamental pharmacological action of anticholinergics.
Administration And Dosage
Dogs
Glycopyrrolate Injectable may be administered intravenously, intramuscularly or subcutaneously at the rate of 5 micrograms/lb body weight (0.25 mL per 10 lbs body weight).Cats
Glycopyrrolate Injectable may be administered intramuscularly at the rate of 5 micrograms/lb body weight (0.25 mL per 10 lbs body weight). For maximum anticholinergic effect administer Glycopyrrolate Injectable 15 minutes prior to anesthetic administration in cats.How Supplied
Glycopyrrolate Injectable is supplied in 20 mL vials (0.2 mg/mL).
NDC 59130-754-13 - 20 mL Vial - 0.2 mg/mL.
Store between 15° and 30°C (59° and 86°F).
References
(1) Short, Charles E.; Paddleford, Robert R.; Cloyd, Grover D. Glycopyrrolate for prevention of pulmonary complications during anesthesia. Modern Veterinary Practice, May, 1974.
AmTech® is a registered trademark of IVX Animal Health, Inc.
Manufactured By: IVX Animal Health, Inc., St. Joseph, MO 64503
|
Net Contents: |
NDC |
|
|
20 mL |
59130-754-13 |
600117-13 C-ISS0606 |
Nac No.
10740961formerly known as IVX Animal Health, Inc.
3915 S. 48TH ST. TERRACE, P.O. BOX 8039 (64508), ST. JOSEPH, MO, 64503
| Telephone: | 800-759-3664 | |
| Fax: | 816-676-6873 | |
| Website: | www.tevaanimalhealth.com |
 |
Every effort has been made to ensure the accuracy of the AmTech Glycopyrrolate Injectable information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2025 Animalytix LLC. Updated: 2025-05-29
